Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition
- PMID: 22282653
- PMCID: PMC8543151
- DOI: 10.1158/0008-5472.CAN-11-2905
Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition
Abstract
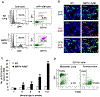
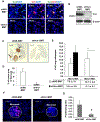
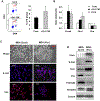
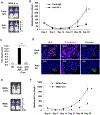
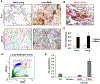
Tumors systemically initiate metastatic niches in distant target metastatic organs. These niches, composed of bone marrow-derived hematopoietic cells, provide permissive conditions for future metastases. However, the mechanisms by which these cells mediate outgrowth of metastatic tumor cells are not completely known. Using mouse models of spontaneous breast cancer, we show enhanced recruitment of bone marrow-derived CD11b(+)Gr1(+) myeloid progenitor cells in the premetastatic lungs. Gene expression profiling revealed that the myeloid cells from metastatic lungs express versican, an extracellular matrix proteoglycan. Notably, versican in metastatic lungs was mainly contributed by the CD11b(+)Ly6C(high) monocytic fraction of the myeloid cells and not the tumor cells or other stromal cells. Versican knockdown in the bone marrow significantly impaired lung metastases in vivo, without impacting their recruitment to the lungs or altering the immune microenvironment. Versican stimulated mesenchymal to epithelial transition of metastatic tumor cells by attenuating phospho-Smad2 levels, which resulted in elevated cell proliferation and accelerated metastases. Analysis of clinical specimens showed elevated versican expression within the metastatic lung of patients with breast cancer. Together, our findings suggest that selectively targeting tumor-elicited myeloid cells or versican represents a potential therapeutic strategy for combating metastatic disease.
Figures

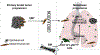
References
-
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5:1744–50. - PubMed
-
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–25. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

