PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer
- PMID: 22282656
- PMCID: PMC5080984
- DOI: 10.1158/0008-5472.CAN-11-2602
PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer
Abstract
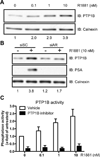
The androgen receptor (AR) signaling axis plays a key role in the pathogenesis of prostate cancer. In this study, we found that the protein tyrosine phosphatase PTP1B, a well-established regulator of metabolic signaling, was induced after androgen stimulation of AR-expressing prostate cancer cells. PTP1B induction by androgen occurred at the mRNA and protein levels to increase PTP1B activity. High-resolution chromosome mapping revealed AR recruitment to two response elements within the first intron of the PTP1B encoding gene PTPN1, correlating with an AR-mediated increase in RNA polymerase II recruitment to the PTPN1 transcriptional start site. We found that PTPN1 and AR genes were coamplified in metastatic tumors and that PTPN1 amplification was associated with a subset of high-risk primary tumors. Functionally, PTP1B depletion delayed the growth of androgen-dependent human prostate tumors and impaired androgen-induced cell migration and invasion in vitro. However, PTP1B was also required for optimal cell migration of androgen-independent cells. Collectively, our results established the AR as a transcriptional regulator of PTPN1 transcription and implicated PTP1B in a tumor-promoting role in prostate cancer. Our findings support the preclinical testing of PTP1B inhibitors for prostate cancer treatment.
Figures

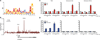


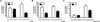
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. - PubMed
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

