Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation
- PMID: 22283603
- PMCID: PMC3277931
- DOI: 10.1089/cbr.2012.1202
Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation
Abstract
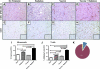
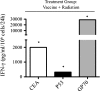
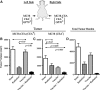
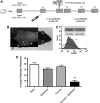
Radiation is a primary modality in cancer treatment. Radiation can also reduce tumor growth outside the treatment field, often referred to as the abscopal effect. The mechanisms and therapeutic potential of the abscopal effect have not been fully elucidated. We evaluated the role of vaccination directed against a tumor-associated antigen (TAA) in the induction and amplification of radiation induced abscopal effects. Active-specific immunotherapy with a TAA-specific vaccine regimen was used to induce and potentiate T-cell responses against carcinoembryonic antigen (CEA) in combination with local irradiation of subcutaneous tumors. We examined the potential synergy of a poxvirus-based CEA vaccine regimen in CEA-transgenic (Tg) mice in combination with either external beam radiation or brachytherapy of local tumors. The induction of CD8(+) T cells specific for multiple TAAs not encoded by the vaccine was observed after the combination therapy. In two tumor models, the antigen cascade responses induced by vaccine and local irradiation mediated the regression of antigen negative metastases at distal subcutaneous or pulmonary sites. Clinically, local control of the primary tumor is necessary and can sometimes prevent metastases; however, irradiation generally fails to control preexisting metastases. These studies suggest that by coupling tumor irradiation with immunotherapy, the abscopal effect can transcend from anecdotal observation to a defined mechanism that can be exploited for the treatment of systemic disease.
Figures



References
-
- Blyth BJ. Sykes PJ. Radiation-induced bystander effects: What are they, and how relevant are they to human radiation exposures? Radiat Res. 2011;176:139. - PubMed
-
- Kaminski JM. Shinohara E. Summers JB, et al. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159. - PubMed
-
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234. - PubMed
-
- Eades-Perner AM. van der Putten H. Hirth A, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169. - PubMed
-
- Robbins PF. Kantor JA. Salgaller M, et al. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

