Discovery of potent and selective covalent inhibitors of JNK
- PMID: 22284361
- PMCID: PMC3270411
- DOI: 10.1016/j.chembiol.2011.11.010
Discovery of potent and selective covalent inhibitors of JNK
Abstract
The mitogen-activated kinases JNK1/2/3 are key enzymes in signaling modules that transduce and integrate extracellular stimuli into coordinated cellular response. Here, we report the discovery of irreversible inhibitors of JNK1/2/3. We describe two JNK3 cocrystal structures at 2.60 and 2.97 Å resolution that show the compounds form covalent bonds with a conserved cysteine residue. JNK-IN-8 is a selective JNK inhibitor that inhibits phosphorylation of c-Jun, a direct substrate of JNK, in cells exposed to submicromolar drug in a manner that depends on covalent modification of the conserved cysteine residue. Extensive biochemical, cellular, and pathway-based profiling establish the selectivity of JNK-IN-8 for JNK and suggests that the compound will be broadly useful as a pharmacological probe of JNK-dependent signal transduction. Potential lead compounds have also been identified for kinases, including IRAK1, PIK3C3, PIP4K2C, and PIP5K3.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

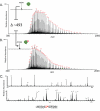
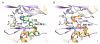



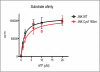

References
-
- Aguirre V, Uchida T, Yenush L, Davis RJ, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000;275:9047–9054. - PubMed
-
- Aguirre V, Werner ED, Giraud J, Lee Y, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. - PubMed
-
- Alam M, Beevers RE, Ceska T, Davenport RJ, Dickson KM, Fortunato M, Gowers L, Haughan AF, James LA, Jones MW, et al. Synthesis and SAR of aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg. Med. Chem. Lett. 2007;17:3463–3467. - PubMed
-
- Angell RM, Atkinson FL, Brown MJ, Chuang TT, Christopher JA, Cichy-Knight M, Dunn AK, Hightower KE, Malkakorpi S, Musgrave JR, et al. N-(3-Cyano-4,5,6,7-tetrahydro-1-benzothien-2-yl)amides as potent, selective, inhibitors of JNK2 and JNK3. Bioorg. Med. Chem. Lett. 2007;17:1296–1301. - PubMed
-
- Atwell S, Adams JM, Badger J, Buchanan MD, Feil IK, Froning KJ, Gao X, Hendle J, Keegan K, Leon BC, Muller-Deickmann HJ, et al. A novel mode of Gleevec binding is revealed by the structure of spleen tyrosine kinase. J. Biol. Chem. 2004;279:55827–55832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS057153-04/NS/NINDS NIH HHS/United States
- U54 HG006097/HG/NHGRI NIH HHS/United States
- RC2 CA148164/CA/NCI NIH HHS/United States
- U01 NS057153/NS/NINDS NIH HHS/United States
- 1RC2HG005693/HG/NHGRI NIH HHS/United States
- MC_G1000735/MRC_/Medical Research Council/United Kingdom
- 089698/WT_/Wellcome Trust/United Kingdom
- MC_U127070193/MRC_/Medical Research Council/United Kingdom
- 5 RCD HG005693-02/HG/NHGRI NIH HHS/United States
- RC2 HG005693/HG/NHGRI NIH HHS/United States
- 5 RC2 CA148164-02/CA/NCI NIH HHS/United States
- 1 U54 HG 006907-01/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

