B-1 B cell development in the fetus and adult
- PMID: 22284417
- PMCID: PMC3269035
- DOI: 10.1016/j.immuni.2011.11.017
B-1 B cell development in the fetus and adult
Abstract
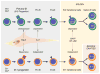
Models of hematopoiesis often depict lymphocyte production as a uniform process in which a homogenous population of hematopoietic stem cells (HSCs) generates progenitors from which all types of lymphocytes are derived. However, it is increasingly evident that these schemes are too simplistic and that the lymphoid potential of HSCs and precursors arising in the embryo, fetus, neonate, and adult is remarkably distinct. We review recent findings regarding the development of B lymphocytes, and the B-1 B cell lineage in particular, as a case in point. These studies show that B-1 and B-2 B cells involved in innate and adaptive immune responses, respectively, arise in staggered waves of development from distinct progenitors. We discuss the implications of this layered model of B cell development for understanding normal and dysregulated B lymphopoiesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. - PubMed
-
- Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. - PubMed
-
- Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. - PubMed
-
- Antin J, Emerson S, Martin P, Gadol N, Ault K. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986;136:505–510. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

