Scd6 targets eIF4G to repress translation: RGG motif proteins as a class of eIF4G-binding proteins
- PMID: 22284680
- PMCID: PMC3277450
- DOI: 10.1016/j.molcel.2011.11.026
Scd6 targets eIF4G to repress translation: RGG motif proteins as a class of eIF4G-binding proteins
Abstract
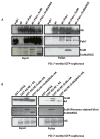
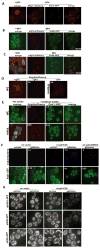
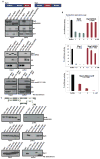
The formation of mRNPs controls the interaction of the translation and degradation machinery with individual mRNAs. The yeast Scd6 protein and its orthologs regulate translation and mRNA degradation in yeast, C. elegans, D. melanogaster, and humans by an unknown mechanism. We demonstrate that Scd6 represses translation by binding the eIF4G subunit of eIF4F in a manner dependent on its RGG domain, thereby forming an mRNP repressed for translation initiation. Strikingly, several other RGG domain-containing proteins in yeast copurify with eIF4E/G and we demonstrate that two such proteins, Npl3 and Sbp1, also directly bind eIF4G and repress translation in a manner dependent on their RGG motifs. These observations identify the mechanism of Scd6 function through its RGG motif and indicate that eIF4G plays an important role as a scaffolding protein for the recruitment of translation repressors.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Abramoff M, Magelhaes P, Ram S. Image processing with ImageJ. Biophotonics Intl. 2004;11:36–42.
-
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10(6):430–6. - PubMed
-
- Barbee S, Estes P, Cziko A, Hillebrand J, Luedeman R, Coller J, Johnson N, Howlett I, Geng C, Ueda R, Brand A, Newbury S, Wilhelm J, Levine R, Nakamura A, Parker R, Ramaswami M. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. - PMC - PubMed
-
- Boag P, Nakamura A, Blackwell K. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development. 2005;132:4975–4986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

