Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles
- PMID: 22285280
- PMCID: PMC3294074
- DOI: 10.1016/j.jaci.2012.01.032
Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles
Abstract
The most profound primary immunodeficiency disease, severe combined immunodeficiency (SCID), is fatal in infancy unless affected infants are provided with an adaptive immune system through allogeneic hematopoietic cell transplantation, enzyme replacement, or gene therapy. However, most infants with SCID lack a family history or any clinical clues before the onset of infections, making this serious but treatable disease a candidate for population-based newborn screening. Of several approaches considered for SCID screening, testing for T-cell receptor excision circles (TRECs), a DNA biomarker of normal T-cell development, has proved successful. TREC numbers can be measured in DNA isolated from the dried bloodspots already routinely collected for newborn screening. Infants with low or absent TRECs can thus be identified and referred for confirmatory testing and prompt intervention. TREC testing of newborns is now being performed in several states, indicating that this addition to the newborn screening panel can be successfully integrated into state public health programs.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures
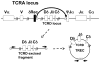
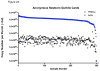

Comment in
-
Reply: To PMID 22285280.J Allergy Clin Immunol. 2013 Apr;131(4):1252-3. doi: 10.1016/j.jaci.2012.09.040. Epub 2013 Jan 12. J Allergy Clin Immunol. 2013. PMID: 23321207 No abstract available.
-
Flow cytometric assessment of cord blood as an alternative strategy for population-based screening of severe combined immunodeficiency.J Allergy Clin Immunol. 2013 Apr;131(4):1251-2. doi: 10.1016/j.jaci.2012.09.039. Epub 2013 Jan 16. J Allergy Clin Immunol. 2013. PMID: 23333079 No abstract available.
Similar articles
-
The case for newborn screening for severe combined immunodeficiency and related disorders.Ann N Y Acad Sci. 2011 Dec;1246:108-17. doi: 10.1111/j.1749-6632.2011.06346.x. Ann N Y Acad Sci. 2011. PMID: 22236435 Free PMC article. Review.
-
Cellular calibrators to quantitate T-cell receptor excision circles (TRECs) in clinical samples.Mol Genet Metab. 2012 Nov;107(3):586-91. doi: 10.1016/j.ymgme.2012.09.018. Epub 2012 Sep 21. Mol Genet Metab. 2012. PMID: 23062576 Free PMC article.
-
Newborn screening for severe combined immunodeficiency using a novel and simplified method to measure T-cell excision circles (TREC).Clin Immunol. 2017 Feb;175:51-55. doi: 10.1016/j.clim.2016.11.016. Epub 2016 Dec 2. Clin Immunol. 2017. PMID: 27916705
-
The Wisconsin approach to newborn screening for severe combined immunodeficiency.J Allergy Clin Immunol. 2012 Mar;129(3):622-7. doi: 10.1016/j.jaci.2011.12.004. Epub 2012 Jan 11. J Allergy Clin Immunol. 2012. PMID: 22244594
-
Newborn Screening for Severe Combined Immunodeficiency.Curr Allergy Asthma Rep. 2018 May 10;18(6):34. doi: 10.1007/s11882-018-0783-9. Curr Allergy Asthma Rep. 2018. PMID: 29749587 Review.
Cited by
-
Nijmegen breakage syndrome detected by newborn screening for T cell receptor excision circles (TRECs).J Clin Immunol. 2015 Feb;35(2):227-33. doi: 10.1007/s10875-015-0136-6. Epub 2015 Feb 13. J Clin Immunol. 2015. PMID: 25677497 Free PMC article.
-
Newborn screening for SCID in New York State: experience from the first two years.J Clin Immunol. 2014 Apr;34(3):289-303. doi: 10.1007/s10875-014-0006-7. Epub 2014 Mar 1. J Clin Immunol. 2014. PMID: 24578017 Free PMC article.
-
Use of tuberculin skin test for assessment of immune recovery among previously malnourished children in Ethiopia.BMC Res Notes. 2017 Nov 7;10(1):570. doi: 10.1186/s13104-017-2909-x. BMC Res Notes. 2017. PMID: 29115985 Free PMC article.
-
The diagnosis of severe combined immunodeficiency: Implementation of the PIDTC 2022 Definitions.J Allergy Clin Immunol. 2023 Feb;151(2):547-555.e5. doi: 10.1016/j.jaci.2022.10.021. Epub 2022 Nov 28. J Allergy Clin Immunol. 2023. PMID: 36456360 Free PMC article.
-
Novel Genome-Editing Tools to Model and Correct Primary Immunodeficiencies.Front Immunol. 2015 May 21;6:250. doi: 10.3389/fimmu.2015.00250. eCollection 2015. Front Immunol. 2015. PMID: 26052330 Free PMC article. Review.
References
-
- Puck JM. X-linked severe combined immunodeficiency. In: Ochs H, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. 2. NY (NY): Oxford Univ Press; 2007. pp. 123–36.
-
- Gatti RA, Meuwissen HJ, Allen Hd, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2(7583):1366–9. - PubMed
-
- O’Reilly RJ, Friedrich W, Small TN. Transplantation approaches for severe combined immunodeficiency disease, Wiskott-Aldrich syndrome, and other lethal genetic combined immunodeficiency disorders. In: Forman SJ, Blume KG, Thomas ED, editors. Bone marrow transplantation. Boston (MA): Blackwell Scientific Publications; 1994. pp. 849–67.
-
- Buckley RH, Schiff RI, Schiff SE, Markert LM, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–87. - PubMed
-
- Knutsen AP, Wall DA. Umbilical cord blood transplantation in severe T-cell immunodeficiency disorders: two-year experience. J Clin Immunol. 2000;20:466–76. - PubMed

