Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori
- PMID: 22285806
- PMCID: PMC3335966
- DOI: 10.1053/j.gastro.2012.01.029
Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori
Abstract
Background & aims: Macrophages mediate the epithelial response to Helicobacter pylori and are involved in the development of gastritis. Sonic Hedgehog (Shh) regulates gastric epithelial differentiation and function, but little is known about its immunoregulatory role in the stomach. We investigated whether gastric Shh acts as a macrophage chemoattractant during the innate immune response to H pylori infection.
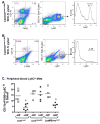
Methods: Mice with parietal cell-specific deletion of Shh (PC-Shh(KO)) and control mice were infected with H pylori. Levels of gastric Shh, cytokines, and chemokines were assayed by quantitative reverse-transcriptase polymerase chain reaction or by a Luminex-based multiplex assay 2, 7, or 180 days after infection. Circulating concentrations of Shh were measured by enzyme-linked immunosorbent assay. Bone marrow chimera experiments were performed with mice that have myeloid cell-specific deletion of the Hedgehog signal transduction protein Smoothened (LysMCre/Smo(KO)). Macrophage recruitment was measured in gastric tissue and peripheral blood by fluorescence-activated cell sorting analysis.
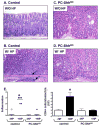
Results: Control mice infected with H pylori for 6 months developed an inflammatory response characterized by infiltration of CD4(+) T cells and increased levels of interferon gamma and interleukin 1β in the stomach. PC-Shh(KO) mice did not develop gastritis, even after 6 months of infection with H pylori. Control mice had increased concentrations of Shh, accompanied by the recruitment of CD11b(+)F4/80(+)Ly6C(high) macrophages 2 days after infection. Control mice that received bone marrow transplants from control mice had an influx of macrophages to the gastric mucosa in response to H pylori infection; this was not observed in H pylori-infected control mice that received bone marrow transplants from LysMCre/Smo(KO) mice.
Conclusions: H pylori induces release of Shh from the stomach; Shh acts as a macrophage chemoattractant during initiation of gastritis.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Mattapallil JJ, Dandekar S, Canfield DR, Solnick JV. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000;118:307–15. - PubMed
-
- Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–61. - PubMed
-
- Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. - PubMed
-
- Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51:577–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

