Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo
- PMID: 22286008
- PMCID: PMC3348446
- DOI: 10.1016/j.jconrel.2012.01.009
Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo
Abstract
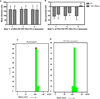
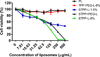
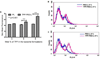
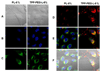
Previously, stearyl triphenylphosphonium (STPP)-modified liposomes (STPP-L) were reported to target mitochondria. To overcome a non-specific cytotoxicity of STPP-L, we synthesized a novel polyethylene glycol-phosphatidylethanolamine (PEG-PE) conjugate with the TPP group attached to the distal end of the PEG block (TPP-PEG-PE). This conjugate was incorporated into the liposomal lipid bilayer, and the modified liposomes were studied for their toxicity, mitochondrial targeting, and efficacy in delivering paclitaxel (PTX) to cancer cells in vitro and in vivo. These TPP-PEG-PE-modified liposomes (TPP-PEG-L), surface grafted with as high as 8 mol% of the conjugate, were less cytotoxic compared to STPP-L or PEGylated STPP-L. At the same time, TPP-PEG-L demonstrated efficient mitochondrial targeting in cancer cells as shown by confocal microscopy in co-localization experiments with stained mitochondria. PTX-loaded TPP-PEG-L demonstrated enhanced PTX-induced cytotoxicity and anti-tumor efficacy in cell culture and mouse experiments compared to PTX-loaded unmodified plain liposomes (PL). Thus, TPP-PEG-PE can serve as a targeting ligand to prepare non-toxic liposomes as mitochondria-targeted drug delivery systems (DDS).
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures



References
-
- Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. - PubMed
-
- Shi F, Hoekstra D. Effective intracellular delivery of oligonucleotides in order to make sense of antisense. J Control Release. 2004;97:189–209. - PubMed
-
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. - PubMed
-
- Stefano JE, Hou L, Honey D, Kyazike J, Park A, Zhou Q, Pan CQ, Edmunds T. In vitro and in vivo evaluation of a non-carbohydrate targeting platform for lysosomal proteins. J Control Release. 2009;135:113–118. - PubMed
-
- Won YW, Lim KS, Kim YH. Intracellular organelle-targeted non-viral gene delivery systems. J Control Release. 2011;152:99–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

