Dynamic monitoring of glucagon secretion from living cells on a microfluidic chip
- PMID: 22286080
- PMCID: PMC3324330
- DOI: 10.1007/s00216-012-5755-7
Dynamic monitoring of glucagon secretion from living cells on a microfluidic chip
Abstract
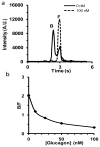
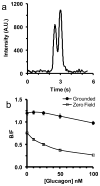
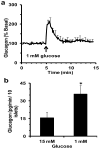
A rapid microfluidic based capillary electrophoresis immunoassay (CEIA) was developed for on-line monitoring of glucagon secretion from pancreatic islets of Langerhans. In the device, a cell chamber containing living islets was perfused with buffers containing either high or low glucose concentration. Perfusate was continuously sampled by electroosmosis through a separate channel on the chip. The perfusate was mixed on-line with fluorescein isothiocyanate-labeled glucagon (FITC-glucagon) and monoclonal anti-glucagon antibody. To minimize sample dilution, the on-chip mixing ratio of sampled perfusate to reagents was maximized by allowing reagents to only be added by diffusion. Every 6 s, the reaction mixture was injected onto a 1.5-cm separation channel where free FITC-glucagon and the FITC-glucagon-antibody complex were separated under an electric field of 700 V cm(-1). The immunoassay had a detection limit of 1 nM. Groups of islets were quantitatively monitored for changes in glucagon secretion as the glucose concentration was decreased from 15 to 1 mM in the perfusate revealing a pulse of glucagon secretion during a step change. The highly automated system should be enable studies of the regulation of glucagon and its potential role in diabetes and obesity. The method also further demonstrates the potential of rapid CEIA on microfluidic systems for monitoring cellular function.
Figures
References
-
- El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442:403–411. - PubMed
-
- Wlodkowic D, Cooper JM. Curr Opin Chem Biol. 2010;14:556–567. - PubMed
-
- Salieb-Beugelaar GB, Simone G, Arora A, Philippi A, Manz A. Anal Chem. 2010;82:4848–4864. - PubMed
-
- Cheng W, Klauke N, Smith G, Cooper JM. Electrophoresis. 2010;31:1405–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

