Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis
- PMID: 22286218
- PMCID: PMC3288335
- DOI: 10.1038/ng.1076
Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis
Abstract
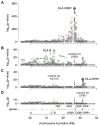
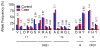
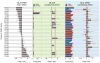
The genetic association of the major histocompatibility complex (MHC) to rheumatoid arthritis risk has commonly been attributed to alleles in HLA-DRB1. However, debate persists about the identity of the causal variants in HLA-DRB1 and the presence of independent effects elsewhere in the MHC. Using existing genome-wide SNP data in 5,018 individuals with seropositive rheumatoid arthritis (cases) and 14,974 unaffected controls, we imputed and tested classical alleles and amino acid polymorphisms in HLA-A, HLA-B, HLA-C, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DQB1 and HLA-DRB1, as well as 3,117 SNPs across the MHC. Conditional and haplotype analyses identified that three amino acid positions (11, 71 and 74) in HLA-DRβ1 and single-amino-acid polymorphisms in HLA-B (at position 9) and HLA-DPβ1 (at position 9), which are all located in peptide-binding grooves, almost completely explain the MHC association to rheumatoid arthritis risk. This study shows how imputation of functional variation from large reference panels can help fine map association signals in the MHC.
Figures


Comment in
-
From HLA association to function.Nat Genet. 2012 Feb 27;44(3):235-6. doi: 10.1038/ng.2207. Nat Genet. 2012. PMID: 22366857
-
Rheumatoid arthritis: RA risk variants in the groove.Nat Rev Rheumatol. 2012 Mar 2;8(3):121. doi: 10.1038/nrrheum.2012.18. Nat Rev Rheumatol. 2012. PMID: 22388696 No abstract available.
References
-
- Isenberg D. Oxford textbook of rheumatology. Oxford University Press; Oxford; New York: 2004. p. 1278.
-
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–72. - PubMed
-
- van der Woude D, et al. Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum. 2009;60:916–23. - PubMed
-
- Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

