Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation
- PMID: 22286766
- PMCID: PMC3343184
- DOI: 10.1038/onc.2011.656
Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation
Abstract
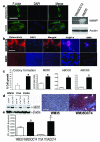
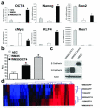
There is enormous interest to target cancer stem cells (CSCs) for clinical treatment because these cells are highly tumorigenic and resistant to chemotherapy. Oct4 is expressed by CSC-like cells in different types of cancer. However, function of Oct4 in tumor cells is unclear. In this study, we showed that expression of Oct4 gene or transmembrane delivery of Oct4 protein promoted dedifferentiation of melanoma cells to CSC-like cells. The dedifferentiated melanoma cells showed significantly decreased expression of melanocytic markers and acquired the ability to form tumor spheroids. They showed markedly increased resistance to chemotherapeutic agents and hypoxic injury. In the subcutaneous xenograft and tail vein injection assays, these cells had significantly increased tumorigenic capacity. The dedifferentiated melanoma cells acquired features associated with CSCs such as multipotent differentiation capacity and expression of melanoma CSC markers such as ABCB5 and CD271. Mechanistically, Oct4-induced dedifferentiation was associated with increased expression of endogenous Oct4, Nanog and Klf4, and global gene expression changes that enriched for transcription factors. RNAi-mediated knockdown of Oct4 in dedifferentiated cells led to diminished CSC phenotypes. Oct4 expression in melanoma was regulated by hypoxia and its expression was detected in a sub-population of melanoma cells in clinical samples. Our data indicate that Oct4 is a positive regulator of tumor dedifferentiation. The results suggest that CSC phenotype is dynamic and may be acquired through dedifferentiation. Oct4-mediated tumor cell dedifferentiation may have an important role during tumor progression.
Figures





References
-
- Bennett DC. Differentiation in mouse melanoma cells: initial reversibility and an on-off stochastic model. Cell. 1983;34:445–453. - PubMed
-
- Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. - PubMed
-
- Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283:17969–17978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

