Structure and ligand recognition of class C GPCRs
- PMID: 22286915
- PMCID: PMC4077135
- DOI: 10.1038/aps.2011.186
Structure and ligand recognition of class C GPCRs
Abstract
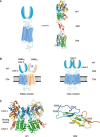
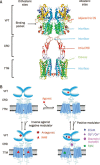
The G-protein-coupled receptors (GPCRs) are one of the largest super families of cell-surface receptors and play crucial roles in virtually every organ system. One particular family of GPCRs, the class C GPCRs, is distinguished by a characteristically large extracellular domain and constitutive dimerization. The structure and activation mechanism of this family result in potentially unique ligand recognition sites, thereby offering a variety of possibilities by which receptor activity might be modulated using novel compounds. In the present article, we aim to provide an overview of the exact sites and structural features involved in ligand recognition of the class C GPCRs. Furthermore, we demonstrate the precise steps that occur during the receptor activation process, which underlie the possibilities by which receptor function may be altered by different approaches. Finally, we use four typical family members to illustrate orthosteric and allosteric sites with representative ligands and their corresponding therapeutic potential.
Figures

References
-
- George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–20. - PubMed
-
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. - PubMed
-
- Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–54. - PubMed
-
- Rondard P, Goudet C, Kniazeff J, Pin JP, Prezeau L. The complexity of their activation mechanism opens new possiblities for the modulation of mGlu and GABABclass C G protein-coupled receptors. Neuropharmacology. 2011;60:82–92. - PubMed
-
- Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011;63:59–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

