Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands
- PMID: 22286996
- PMCID: PMC3302589
- DOI: 10.1128/AEM.07050-11
Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands
Abstract
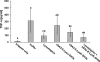
Staphylococci cause bovine mastitis, with Staphylococcus aureus being responsible for the majority of the mastitis-based losses to the dairy industry (up to $2 billion/annum). Treatment is primarily with antibiotics, which are often ineffective and potentially contribute to resistance development. Bacteriophage endolysins (peptidoglycan hydrolases) present a promising source of alternative antimicrobials. Here we evaluated two fusion proteins consisting of the streptococcal λSA2 endolysin endopeptidase domain fused to staphylococcal cell wall binding domains from either lysostaphin (λSA2-E-Lyso-SH3b) or the staphylococcal phage K endolysin, LysK (λSA2-E-LysK-SH3b). We demonstrate killing of 16 different S. aureus mastitis isolates, including penicillin-resistant strains, by both constructs. At 100 μg/ml in processed cow milk, λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b reduced the S. aureus bacterial load by 3 and 1 log units within 3 h, respectively, compared to a buffer control. In contrast to λSA2-E-Lyso-SH3b, however, λSA2-E-LysK-SH3b permitted regrowth of the pathogen after 1 h. In a mouse model of mastitis, infusion of 25 μg of λSA2-E-Lyso-SH3b or λSA2-E-LysK-SH3b into mammary glands reduced S. aureus CFU by 0.63 or 0.81 log units, compared to >2 log for lysostaphin. Both chimeras were synergistic with lysostaphin against S. aureus in plate lysis checkerboard assays. When tested in combination in mice, λSA2-E-LysK-SH3b and lysostaphin (12.5 μg each/gland) caused a 3.36-log decrease in CFU. Furthermore, most protein treatments reduced gland wet weights and intramammary tumor necrosis factor alpha (TNF-α) concentrations, which serve as indicators of inflammation. Overall, our animal model results demonstrate the potential of fusion peptidoglycan hydrolases as antimicrobials for the treatment of S. aureus-induced mastitis.
Figures
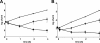
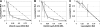
References
-
- Anderson JC, Craven N. 1984. Assessment in the mouse of cefoperazone as a treatment for mastitis. Vet. Rec. 114:607–612 - PubMed
-
- Anderson JC, Heneghan DJ. 1979. Extrapolation from experimental chronic staphylococcal mastitis in mice to experimental infections in cattle. Br. Vet. J. 135:527–535 - PubMed
-
- Becker SC, Foster-Frey J, Donovan DM. 2008. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 287:185–191 - PubMed
-
- Becker SC, Foster-Frey J, Powell A, Kerr D, Donovan DM. 2011. Lysostaphin: molecular changes that preserve staphylolytic activity, p 18–22. In Mendez-Vilas A. (ed), Proceedings of the International Conference on Antimicrobial Research. World Scientific Publishing Co., Singapore
-
- Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM. 2009. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 443:32–41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

