Synthesis of sansalvamide A peptidomimetics: triazole, oxazole, thiazole, and pseudoproline containing compounds
- PMID: 22287031
- PMCID: PMC3266378
- DOI: 10.1016/j.tet.2011.11.089
Synthesis of sansalvamide A peptidomimetics: triazole, oxazole, thiazole, and pseudoproline containing compounds
Abstract
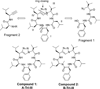
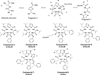
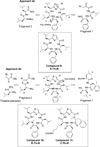
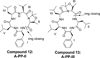
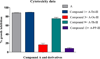
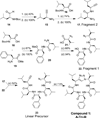
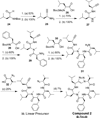
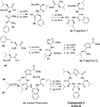
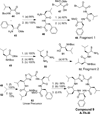
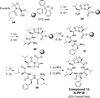
Peptidomimetic-based macrocycles typically have improved pharmacokinetic properties over those observed with peptide analogs. Described are the syntheses of 13 peptidomimetic derivatives that are based on active Sansalvamide A structures, where these analogs incorporate heterocycles (triazoles, oxazoles, thiazoles, or pseudoprolines) along the macrocyclic backbone. The syntheses of these derivatives employ several approaches that can be applied to convert a macrocyclic peptide into its peptidomimetic counterpart. These approaches include peptide modifications to generate the alkyne and azide for click chemistry, a serine conversion into an oxazole, a Hantzsch reaction to generate the thiazole, and protected threonine to generate the pseudoproline derivatives. Furthermore, we show that two different peptidomimetic moieties, triazoles and thiazoles, can be incorporated into the macrocyclic backbone without reducing cytotoxicity: triazole and thiazole.
Figures

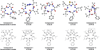

References
-
- Otrubova K, Lushington G, Vander Velde D, McGuire KL, McAlpine SR. Journal of Medicinal Chemistry. 2008;51:530–544. - PubMed
-
- Belofsky GN, Jensen PR, Fenical W. Tetrahedron Lett. 1999;40:2913–2916.
-
- Otrubova K, Lushington GH, Vander Velde D, McGuire KL, McAlpine SR. J. Med. Chem. 2008;51:530–544. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
