Targeting the enhancer of zeste homologue 2 in medulloblastoma
- PMID: 22287205
- PMCID: PMC3375399
- DOI: 10.1002/ijc.27455
Targeting the enhancer of zeste homologue 2 in medulloblastoma
Abstract
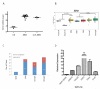
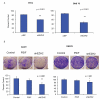
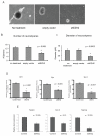
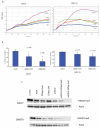
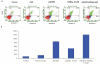
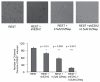
Enhancer of zeste homologue 2 (EZH2) is the catalytic subunit of Polycomb repressive complex 2 that catalyzes the trimethylation of histone H3 on Lys 27, and represses gene transcription. EZH2 enhances cancer-cell proliferation and regulates stem cell maintenance and differentiation. Here, we demonstrate that EZH2 is highly expressed in medulloblastoma, a highly malignant brain tumor of childhood, and this altered expression is correlated with genomic gain of chromosome 7 in a subset of medulloblastoma. Inhibition of EZH2 by RNAi suppresses medulloblastoma tumor cell growth. We show that 3-deazaneplanocin A, a chemical inhibitor of EZH2, can suppress medulloblastoma cell growth partially by inducing apoptosis. Suppression of EZH2 expression diminishes the ability of tumor cells to form spheres in culture and strongly represses the ability of known oncogenes to transform neural stem cells. These findings establish a role of EZH2 in medulloblastoma and identify EZH2 as a potential therapeutic target especially in high-risk tumors.
Copyright © 2012 UICC.
Figures
References
-
- Dhall G. Medulloblastoma. J Child Neurol. 2009;24:1418–30. - PubMed
-
- Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Archives of Neurology. 2008;65:1419–24. - PubMed
-
- Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley DM, Tyc VL, Kun L, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–9. - PubMed
-
- Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

