Timing of posterior parahippocampal gyrus activity reveals multiple scene processing stages
- PMID: 22287281
- PMCID: PMC6870532
- DOI: 10.1002/hbm.21515
Timing of posterior parahippocampal gyrus activity reveals multiple scene processing stages
Abstract
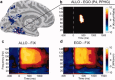
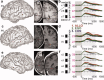
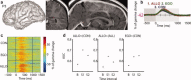
Posterior parahippocampal gyrus (PPHG) is strongly involved during scene recognition and spatial cognition. How PPHG electrophysiological activity could underlie these functions, and whether they share similar timing mechanisms is unknown. We addressed this question in two intracerebral experiments which revealed that PPHG neural activity dissociated an early stimulus-driven effect (>200 and <500 ms) and a late task-related effect (>600 and <800 ms). Strongest PPHG gamma band (50-150 Hz) activities were found early when subjects passively viewed scenes (scene selectivity effect) and lately when they had to estimate the position of an object relative to the environment (allocentric effect). Based on single trial analyses, we were able to predict when patients viewed scenes (compared to other visual categories) and when they performed allocentric judgments (compared to other spatial judgments). The anatomical location corresponding to the strongest effects was in the depth of the collateral sulcus. Our findings directly affect current theories of visual scene processing and spatial orientation by providing new timing constraints and by demonstrating the existence of separable information processing stages in the functionally defined parahippocampal place area.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




Similar articles
-
Temporal components in the parahippocampal place area revealed by human intracerebral recordings.J Neurosci. 2013 Jun 12;33(24):10123-31. doi: 10.1523/JNEUROSCI.4646-12.2013. J Neurosci. 2013. PMID: 23761907 Free PMC article.
-
Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation.J Neurosci. 2004 Nov 10;24(45):10223-8. doi: 10.1523/JNEUROSCI.3373-04.2004. J Neurosci. 2004. PMID: 15537894 Free PMC article.
-
Scene-selective coding by single neurons in the human parahippocampal cortex.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):1153-1158. doi: 10.1073/pnas.1608159113. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096381 Free PMC article.
-
Parahippocampal and retrosplenial contributions to human spatial navigation.Trends Cogn Sci. 2008 Oct;12(10):388-96. doi: 10.1016/j.tics.2008.07.004. Epub 2008 Aug 28. Trends Cogn Sci. 2008. PMID: 18760955 Free PMC article. Review.
-
Making Sense of Real-World Scenes.Trends Cogn Sci. 2016 Nov;20(11):843-856. doi: 10.1016/j.tics.2016.09.003. Epub 2016 Oct 18. Trends Cogn Sci. 2016. PMID: 27769727 Free PMC article. Review.
Cited by
-
Identifying task-relevant spectral signatures of perceptual categorization in the human cortex.Sci Rep. 2020 May 12;10(1):7870. doi: 10.1038/s41598-020-64243-6. Sci Rep. 2020. PMID: 32398733 Free PMC article.
-
Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults.Hum Brain Mapp. 2015 Mar;36(3):897-910. doi: 10.1002/hbm.22674. Epub 2014 Nov 6. Hum Brain Mapp. 2015. PMID: 25376125 Free PMC article.
-
How silent is silent reading? Intracerebral evidence for top-down activation of temporal voice areas during reading.J Neurosci. 2012 Dec 5;32(49):17554-62. doi: 10.1523/JNEUROSCI.2982-12.2012. J Neurosci. 2012. PMID: 23223279 Free PMC article.
-
Spatial position information accumulates steadily over time.J Neurosci. 2013 Nov 20;33(47):18396-401. doi: 10.1523/JNEUROSCI.1864-13.2013. J Neurosci. 2013. PMID: 24259564 Free PMC article.
-
Anatomical dissociation of intracerebral signals for reward and punishment prediction errors in humans.Nat Commun. 2021 Jun 7;12(1):3344. doi: 10.1038/s41467-021-23704-w. Nat Commun. 2021. PMID: 34099678 Free PMC article.
References
-
- Aguirre GK, D'Esposito M ( 1999): Topographical disorientation: A synthesis and taxonomy. Brain 122( Pt 9): 1613–1628. - PubMed
-
- Aguirre GK, Zarahn E, D'Esposito M ( 1998): An area within human ventral cortex sensitive to “building” stimuli: Evidence and implications. Neuron 21: 373–383. - PubMed
-
- Bar M ( 2004): Visual objects in context. Nat Rev Neurosci 5: 617–629. - PubMed
-
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB ( 2010): Grid cells in pre‐ and parasubiculum. Nat Neurosci 13: 987–994. - PubMed
-
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L ( 1998): Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36: 1217–1238. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

