Transcriptional and posttranscriptional events control copper-responsive expression of a Rhodobacter capsulatus multicopper oxidase
- PMID: 22287514
- PMCID: PMC3318457
- DOI: 10.1128/JB.06274-11
Transcriptional and posttranscriptional events control copper-responsive expression of a Rhodobacter capsulatus multicopper oxidase
Abstract
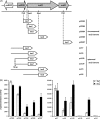
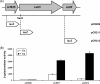
The copper-regulated Rhodobacter capsulatus cutO (multicopper oxidase) gene confers copper tolerance and is carried in the tricistronic orf635-cutO-cutR operon. Transcription of cutO strictly depends on the promoter upstream of orf635, as demonstrated by lacZ reporter fusions to nested promoter fragments. Remarkably, orf635 expression was not affected by copper availability, whereas cutO and cutR were expressed only in the presence of copper. Differential regulation was abolished by site-directed mutations within the orf635-cutO intergenic region, suggesting that this region encodes a copper-responsive mRNA element. Bioinformatic predictions and RNA structure probing experiments revealed an intergenic stem-loop structure as the candidate mRNA element. This is the first posttranscriptional copper response mechanism reported in bacteria.
Figures




Similar articles
-
Maturation of Rhodobacter capsulatus Multicopper Oxidase CutO Depends on the CopA Copper Efflux Pathway and Requires the cutF Product.Front Microbiol. 2021 Sep 8;12:720644. doi: 10.3389/fmicb.2021.720644. eCollection 2021. Front Microbiol. 2021. PMID: 34566924 Free PMC article.
-
The multicopper oxidase CutO confers copper tolerance to Rhodobacter capsulatus.FEMS Microbiol Lett. 2006 Mar;256(1):67-74. doi: 10.1111/j.1574-6968.2005.00094.x. FEMS Microbiol Lett. 2006. PMID: 16487321
-
Molybdate-dependent expression of dimethylsulfoxide reductase in Rhodobacter capsulatus.FEMS Microbiol Lett. 2000 Sep 15;190(2):203-8. doi: 10.1111/j.1574-6968.2000.tb09287.x. FEMS Microbiol Lett. 2000. PMID: 11034280
-
Promoters controlling expression of the alternative nitrogenase and the molybdenum uptake system in Rhodobacter capsulatus are activated by NtrC, independent of sigma54, and repressed by molybdenum.J Bacteriol. 1996 Apr;178(7):2010-7. doi: 10.1128/jb.178.7.2010-2017.1996. J Bacteriol. 1996. PMID: 8606177 Free PMC article.
-
Transcript cleavage, attenuation, and an internal promoter in the Rhodobacter capsulatus puc operon.J Bacteriol. 1999 Aug;181(16):4955-60. doi: 10.1128/JB.181.16.4955-4960.1999. J Bacteriol. 1999. PMID: 10438767 Free PMC article.
Cited by
-
Metabolic Sensing of Extracytoplasmic Copper Availability via Translational Control by a Nascent Exported Protein.mBio. 2023 Feb 28;14(1):e0304022. doi: 10.1128/mbio.03040-22. Epub 2023 Jan 4. mBio. 2023. PMID: 36598193 Free PMC article.
-
Maturation of Rhodobacter capsulatus Multicopper Oxidase CutO Depends on the CopA Copper Efflux Pathway and Requires the cutF Product.Front Microbiol. 2021 Sep 8;12:720644. doi: 10.3389/fmicb.2021.720644. eCollection 2021. Front Microbiol. 2021. PMID: 34566924 Free PMC article.
-
Cu Homeostasis in Bacteria: The Ins and Outs.Membranes (Basel). 2020 Sep 18;10(9):242. doi: 10.3390/membranes10090242. Membranes (Basel). 2020. PMID: 32962054 Free PMC article. Review.
-
Posttranscriptional Regulation by Copper with a New Upstream Open Reading Frame.mBio. 2022 Aug 30;13(4):e0091222. doi: 10.1128/mbio.00912-22. Epub 2022 Jul 13. mBio. 2022. PMID: 35862763 Free PMC article.
-
Complete Genome Sequencing of Polar Arthrobacter sp. PAMC25284, Copper Tolerance Potential Unraveled with Genomic Analysis.Int J Microbiol. 2022 Aug 25;2022:1162938. doi: 10.1155/2022/1162938. eCollection 2022. Int J Microbiol. 2022. PMID: 36061879 Free PMC article.
References
-
- Alexeyev MF. 1995. Three kanamycin resistance gene cassettes with different polylinkers. Biotechniques 18: 52– 55 - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A. 2008. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 7: 209– 216 - PubMed
-
- Arnold W, Pühler A. 1988. A family of high-copy-number plasmid vectors with single end-label sites for rapid nucleotide sequencing. Gene 70: 171– 179 - PubMed
-
- Arredondo M, Núñez MT. 2005. Iron and copper metabolism. Mol. Aspects Med. 26: 313– 327 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

