Altered sinoatrial node function and intra-atrial conduction in murine gain-of-function Scn5a+/ΔKPQ hearts suggest an overlap syndrome
- PMID: 22287583
- PMCID: PMC3330789
- DOI: 10.1152/ajpheart.00357.2011
Altered sinoatrial node function and intra-atrial conduction in murine gain-of-function Scn5a+/ΔKPQ hearts suggest an overlap syndrome
Abstract
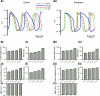
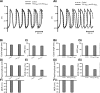
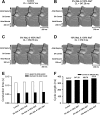
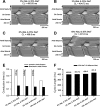
Mutations in SCN5A, the gene encoding the pore-forming subunit of cardiac Na(+) channels, cause a spectrum of arrhythmic syndromes. Of these, sinoatrial node (SAN) dysfunction occurs in patients with both loss- and gain-of-function SCN5A mutations. We explored for corresponding alterations in SAN function and intracardiac conduction and clarified possible mechanisms underlying these in an established mouse long QT syndrome type 3 model carrying a mutation equivalent to human SCN5A-ΔKPQ. Electrophysiological characterizations of SAN function in living animals and in vitro sinoatrial preparations were compared with cellular SAN and two-dimensional tissue models exploring the consequences of Scn5a+/ΔKPQ mutations. Scn5a+/ΔKPQ mice showed prolonged electrocardiographic QT and corrected QT intervals confirming long QT phenotypes. They showed frequent episodes of sinus bradycardia, sinus pause/arrest, and significantly longer sinus node recovery times, suggesting compromised pacemaker activity compared with wild-type mice. Electrocardiographic waveforms suggested depressed intra-atrial, atrioventricular node, and intraventricular conduction in Scn5a+/ΔKPQ mice. Isolated Scn5a+/ΔKPQ sinoatrial preparations similarly showed lower mean intrinsic heart rates and overall slower conduction through the SAN to the surrounding atrium than did wild-type preparations. Computer simulations of both single SAN cells as well as two-dimensional SAN-atrial models could reproduce the experimental observations of impaired pacemaker and sinoatrial conduction in terms of changes produced by both augmented tail and reduced total Na(+) currents, respectively. In conclusion, the gain-of-function long QT syndrome type 3 murine Scn5a+/ΔKPQ cardiac system, in overlap with corresponding features reported in loss-of-function Na(+) channel mutations, shows compromised SAN pacemaker and conduction function explicable in modeling studies through a combination of augmented tail and reduced peak Na(+) currents.
Figures





References
-
- Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine-induced QT interval prolongation: a gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc 77: 413–421, 2002 - PubMed
-
- Baroudi G, Chahine M. Biophysical phenotypes of SCN5A mutations causing long QT and Brugada syndromes. FEBS Lett 487: 224–228, 2000 - PubMed
-
- Beaufort-Krol GC, van den Berg MP, Wilde AA, van Tintelen JP, Viersma JW, Bezzina CR, Bink-Boelkens MT. Developmental aspects of long QT syndrome type 3 and Brugada syndrome on the basis of a single SCN5A mutation in childhood. J Am Coll Cardiol 46: 331–337, 2005 - PubMed
-
- Benhorin J, Taub R, Goldmit M, Kerem B, Kass RS, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation: mutation-specific therapy for long-QT syndrome? Circulation 101: 1698–1706, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

