Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current
- PMID: 22287588
- PMCID: PMC3307380
- DOI: 10.1093/cvr/cvs025
Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current
Abstract
Aims: Remodelling and regional gradients in expression of connexins (Cx) are thought to contribute to atrial electrical dysfunction and atrial fibrillation. We assessed the effect of interaction between Cx43, Cx40, and Cx45 on atrial cell-to-cell coupling and inward Na current (I(Na)) in engineered pairs of atrial myocytes derived from wild-type mice (Cx43(+/+)) and mice with genetic ablation of Cx43 (Cx43(-/-)).
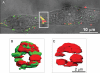
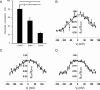
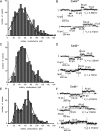
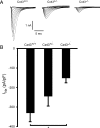
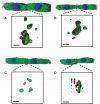
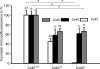
Methods and results: Cell pairs were engineered by microcontact printing from atrial Cx43(+/+) and Cx43(-/-) murine myocytes (1 day before birth, 3-5 days in culture). Dual and single voltage clamp were used to measure intercellular electrical conductance, g(j), and its dependence on transjunctional voltage, V(j), single gap junction channel conductances, and I(Na). 3D reconstructions of Cx43, Cx40, and Cx45 immunosignals in gap junctions were made from confocal slices. Full genetic Cx43 ablation produced a decrease in immunosignals of Cx40 to 62 ± 10% (mean ± SE; n= 17) and Cx45 to 66 ± 8% (n= 16). G(j) decreased from 80 ± 9 nS (Cx43(+/+), n= 17) to 24 ± 2 nS (Cx43(-/-), n= 35). Single channel analysis showed a shift in the main peak of the channel histogram from 49 ± 1.7 nS (Cx43(+/+)) to 67 ± 1.8 nS (Cx43(-/-)) with a second minor peak appearing at 27 ± 1.5 pS. The dependence of g(j) on V(j) decreased with Cx43 ablation. Importantly, peak I(Na) decreased from -350 ± 44 pA/pF (Cx43(+/+)) to -154 ± 28 pA/pF (Cx43(-/-)).
Conclusions: The dependence of Cx40, Cx45, and I(Na) on Cx43 expression indicates a complex interaction between connexins and I(Na) in the atrial intercalated discs that is likely to be of relevance for arrhythmogenesis.
Figures
Similar articles
-
Connexin40 and connexin43 determine gating properties of atrial gap junction channels.J Mol Cell Cardiol. 2010 Jan;48(1):238-45. doi: 10.1016/j.yjmcc.2009.05.014. Epub 2009 May 30. J Mol Cell Cardiol. 2010. PMID: 19486903 Free PMC article.
-
Expression and regulation of connexins in cultured ventricular myocytes isolated from adult rat hearts.Pflugers Arch. 2002 Mar;443(5-6):676-89. doi: 10.1007/s00424-001-0747-z. Epub 2002 Jan 15. Pflugers Arch. 2002. PMID: 11889564
-
Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice.Circ Res. 2004 Jul 23;95(2):170-8. doi: 10.1161/01.RES.0000134923.05174.2f. Epub 2004 Jun 10. Circ Res. 2004. PMID: 15192022
-
Electrical consequences of cardiac myocyte: fibroblast coupling.Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review.
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
Cited by
-
Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications.Pharmacol Rev. 2017 Oct;69(4):396-478. doi: 10.1124/pr.115.012062. Pharmacol Rev. 2017. PMID: 28931622 Free PMC article. Review.
-
Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy.Heart Rhythm. 2013 Mar;10(3):412-9. doi: 10.1016/j.hrthm.2012.11.018. Epub 2012 Nov 23. Heart Rhythm. 2013. PMID: 23178689 Free PMC article.
-
Mechanisms of Arrhythmias in the Brugada Syndrome.Int J Mol Sci. 2020 Sep 25;21(19):7051. doi: 10.3390/ijms21197051. Int J Mol Sci. 2020. PMID: 32992720 Free PMC article. Review.
-
Biomaterials and Culture Systems for Development of Organoid and Organ-on-a-Chip Models.Ann Biomed Eng. 2020 Jul;48(7):2002-2027. doi: 10.1007/s10439-020-02498-w. Epub 2020 Apr 13. Ann Biomed Eng. 2020. PMID: 32285341 Free PMC article. Review.
-
Hypoplastic left heart syndrome (HLHS): molecular pathogenesis and emerging drug targets for cardiac repair and regeneration.Expert Opin Ther Targets. 2021 Aug;25(8):621-632. doi: 10.1080/14728222.2021.1978069. Epub 2021 Sep 15. Expert Opin Ther Targets. 2021. PMID: 34488532 Free PMC article. Review.
References
-
- Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. - PubMed
-
- Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res. 2011;108:294–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

