A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts
- PMID: 22287596
- PMCID: PMC3551213
- DOI: 10.1136/gutjnl-2011-301601
A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts
Abstract
Objective: Wnt signalling is critical for normal intestinal development and homeostasis. Wnt dysregulation occurs in almost all human and murine intestinal tumours and an optimal but not excessive level of Wnt activation is considered favourable for tumourigenesis. The authors assessed effects of pan-intestinal Wnt activation on tissue homeostasis, taking into account underlying physiological Wnt activity and stem-cell number in each region of the bowel.
Design: The authors generated mice that expressed temporally controlled, stabilised β-catenin along the crypt-villus axis throughout the intestines. Physiological Wnt target gene activity was assessed in different regions of normal mouse and human tissue. Human intestinal tumour mutation spectra were analysed.
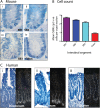
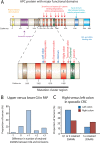
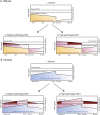
Results: In the mouse, β-catenin stabilisation resulted in a graduated neoplastic response, ranging from dysplastic transformation of the entire epithelium in the proximal small bowel to slightly enlarged crypts of non-dysplastic morphology in the colorectum. In contrast, stem and proliferating cell numbers were increased in all intestinal regions. In the normal mouse and human intestines, stem-cell and Wnt gradients were non-identical, but higher in the small bowel than large bowel in both species. There was also variation in the expression of some Wnt modulators. Human tumour analysis confirmed that different APC mutation spectra are selected in different regions of the bowel.
Conclusions: There are variable gradients in stem-cell number, physiological Wnt activity and response to pathologically increased Wnt signalling along the crypt-villus axis and throughout the length of the intestinal tract. The authors propose that this variation influences regional mutation spectra, tumour susceptibility and lesion distribution in mice and humans.
Conflict of interest statement
Figures


References
-
- Scoville D, Sato T, He X, et al. Current view: intestinal stem cells and signaling. Gastroenterology 2008;134:849–64 - PubMed
-
- Radtke F, Clevers H, Riccio O. From gut homeostasis to cancer. Curr Mol Med 2006;6:275–89 - PubMed
-
- Sieber OM, Tomlinson IP, Lamlum H. The adenomatous polyposis coli (APC) tumour suppressor—genetics, function and disease. Mol Med Today 2000;6:462–9 - PubMed
-
- Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992;359:235–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
