Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia
- PMID: 22287607
- PMCID: PMC3331581
- DOI: 10.1152/ajplung.00196.2011
Vasculoprotective effects of heme oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary dysplasia
Abstract
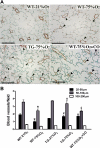
Bronchopulmonary dysplasia (BPD) is characterized by simplified alveolarization and arrested vascular development of the lung with associated evidence of endothelial dysfunction, inflammation, increased oxidative damage, and iron deposition. Heme oxygenase-1 (HO-1) has been reported to be protective in the pathogenesis of diseases of inflammatory and oxidative etiology. Because HO-1 is involved in the response to oxidative stress produced by hyperoxia and is critical for cellular heme and iron homeostasis, it could play a protective role in BPD. Therefore, we investigated the effect of HO-1 in hyperoxia-induced lung injury using a neonatal transgenic mouse model with constitutive lung-specific HO-1 overexpression. Hyperoxia triggered an increase in pulmonary inflammation, arterial remodeling, and right ventricular hypertrophy that was attenuated by HO-1 overexpression. In addition, hyperoxia led to pulmonary edema, hemosiderosis, and a decrease in blood vessel number, all of which were markedly improved in HO-1 overexpressing mice. The protective vascular response may be mediated at least in part by carbon monoxide, due to its anti-inflammatory, antiproliferative, and antiapoptotic properties. HO-1 overexpression, however, did not prevent alveolar simplification nor altered the levels of ferritin and lactoferrin, proteins involved in iron binding and transport. Thus the protective mechanisms elicited by HO-1 overexpression primarily preserve vascular growth and barrier function through iron-independent, antioxidant, and anti-inflammatory pathways.
Figures








References
-
- Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323– 335, 2010 - PubMed
-
- Abman SH, Mourani PM, Sontag M. Bronchopulmonary dysplasia: a genetic disease. Pediatrics 122: 658– 659, 2008 - PubMed
-
- Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA 92: 6798– 6802, 1995 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

