Eculizumab safely reverses neurologic impairment and eliminates need for dialysis in severe atypical hemolytic uremic syndrome
- PMID: 22287852
- PMCID: PMC3262387
- DOI: 10.2147/CPAA.S17904
Eculizumab safely reverses neurologic impairment and eliminates need for dialysis in severe atypical hemolytic uremic syndrome
Abstract
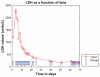
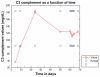
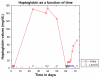
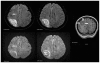
This case report describes how eculizumab reversed neurologic impairment and improved renal damage in severe atypical hemolytic uremic syndrome. A 50-year-old female, after presenting with diarrhea and abdominal pain, developed pancolitis, acute renal failure, and thrombocytopenia. The patient underwent total abdominal colectomy. Pathology confirmed ischemic colitis with scattered mesenteric microthrombi. Due to mental and respiratory decline, she remained intubated. Continuous venovenous hemodialysis was initiated. Renal failure, neurologic changes, hemolysis, thrombotic microangiopathy, and low complement levels all suggested atypical hemolytic uremic syndrome. Eculizumab 900 mg was administered intravenously on hospital day 6 and continued weekly for four doses followed by maintenance therapy. She recovered neurologically and renally after the third dose, and hematologically by the sixth dose. Her recovery has been sustained on long-term eculizumab treatment. In severe atypical hemolytic uremic syndrome, eculizumab safely reverses neurologic impairment and eliminates the need for dialysis. The optimal duration of treatment with eculizumab remains to be determined.
Keywords: atypical hemolytic uremic syndrome; eculizumab; thrombotic microangiopathy.
Figures


References
-
- Chatelet V, Lobbedez T, Fremeaux-Bacchi V, Ficheux M, Ryckelynck JP, Hurault de Ligny B. Eculizumab: Safety and efficacy after 17 months of treatment in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome: Case report. Transplant Proc. 2010;42(10):4353–4355. - PubMed
-
- Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis. 2010;55(44):708–711. - PubMed
-
- ClinicalTrials.gov NCT00838513. [Accessed April 7,2010]. Available from http://clinicaltrials.gov/ct2/show/NCT00838513?term=nct00838513&rank=1.
-
- Muus P, Legendre C, Douglas K, et al. Safety and efficacy of eculizumab in aHUS patients on chronic plasma therapy: Interim analysis from a Phase II trial. Abstract presented at the 43rd annual meeting of the American Society of Nephrology; Denver, CO. November 16–21, 2010; [Accessed April 7, 2010]. Available from: http://www.abstracts2view.com/asn/view.php?nu=ASN10L1_157a&terms.
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

