Nonhuman primate models of Alzheimer-like cerebral proteopathy
- PMID: 22288403
- PMCID: PMC3381739
- DOI: 10.2174/138161212799315885
Nonhuman primate models of Alzheimer-like cerebral proteopathy
Abstract
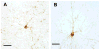
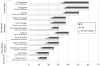
Nonhuman primates are useful for the study of age-associated changes in the brain and behavior in a model that is biologically proximal to humans. The Aβ and tau proteins, two key players in the pathogenesis of Alzheimer's disease (AD), are highly homologous among primates. With age, all nonhuman primates analyzed to date develop senile (Aβ) plaques and cerebral β-amyloid angiopathy. In contrast, significant tauopathy is unusual in simians, and only humans manifest the profound tauopathy, neuronal degeneration and cognitive impairment that characterize Alzheimer's disease. Primates thus are somewhat paradoxical models of AD-like pathology; on the one hand, they are excellent models of normal aging and naturally occurring Aβ lesions, and they can be useful for testing diagnostic and therapeutic agents targeting aggregated forms of Aβ. On the other hand, the resistance of monkeys and apes to tauopathy and AD-related neurodegeneration, in the presence of substantial cerebral Aβ deposition, suggests that a comparative analysis of human and nonhuman primates could yield informative clues to the uniquely human predisposition to Alzheimer's disease.
Figures





References
-
- Carter MD, Simms GA, Weaver DF. The development of new therapeutics for Alzheimer's disease. Clin Pharmacol Ther. 2010;88:475–86. - PubMed
-
- Hauw JJ, Duyckaerts C. Alzheimer's Disease. Oxford University Press; New York: 2001.
-
- Alzheimer A. A characteristic disease of the cerebralcortex. Allegemeine Zeitschrift fur Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;LXIV:146.
-
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

