The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice
- PMID: 22288514
- PMCID: PMC3270336
- DOI: 10.1111/j.1365-2222.2011.03934.x
The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice
Abstract
Background: Ara h 2 and Ara h 6, co-purified together in a 13-25 kD fraction (Ara h 2/6; 20 kD fraction) on gel filtration chromatography, account for the majority of effector activity in a crude peanut extract (CPE) when assayed with RBL SX-38 cells sensitized with IgE from human peanut allergic sera.
Objectives: To determine if Ara h 2/6 are the primary peanut allergens responsible for allergic reactions in vivo and to determine if Ara h 2/6 would be sufficient to prevent allergic reactions to a complete CPE.
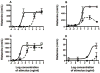
Methods: An oral sensitization mouse model of peanut allergy was used to assess the activity of Ara h 2/6 (20 kD) and CPE without the 20 kD fraction (CPE w/o 20 kD) for allergic provocation challenge and immunotherapy. The activity of these preparations was also tested in an assay of histamine release from human basophils in whole blood.
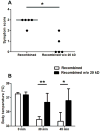
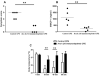
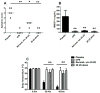
Results: Compared with mice challenged with control CPE, mice challenged with CPE w/o 20 kD experienced reduced symptoms (P < 0.05) and a smaller decrease in body temperature (P < 0.01). Results with the basophil histamine release assay corroborated these findings (P < 0.01). The mouse model was also used to administer Ara h 2/6 (20 kD) in an immunotherapy protocol, in which peanut-allergic mice treated with the 20 kD fraction experienced significantly reduced symptoms, changes in body temperature, and mast cell protease (MMCP-1) release compared with placebo (P < 0.01 for all parameters). Importantly, immunotherapy with the 20 kD fraction was just as effective as treatment with CPE, whereas CPE w/o 20 kD was significantly less effective for higher dose peanut challenges.
Conclusions and clinical relevance: Ara h 2/6 are the most potent peanut allergens in vivo and can be used to desensitize peanut-allergic mice. These results have potential implications for clinical research in the areas of diagnosis and immunotherapy for peanut allergy.
© 2011 Blackwell Publishing Ltd.
Figures



Similar articles
-
Effect of chemical modifications on allergenic potency of peanut proteins.Allergy Asthma Proc. 2015 May-Jun;36(3):185-91. doi: 10.2500/aap.2015.36.3840. Allergy Asthma Proc. 2015. PMID: 25976435 Free PMC article.
-
Blockade of peanut allergy with a novel Ara h 2-Fcγ fusion protein in mice.J Allergy Clin Immunol. 2013 Jan;131(1):213-21.e1-5. doi: 10.1016/j.jaci.2012.10.018. Epub 2012 Nov 27. J Allergy Clin Immunol. 2013. PMID: 23199607 Free PMC article.
-
Contribution of Ara h 2 to peanut-specific, immunoglobulin E-mediated, cell activation.Clin Exp Allergy. 2007 May;37(5):752-63. doi: 10.1111/j.1365-2222.2007.02701.x. Clin Exp Allergy. 2007. PMID: 17456223
-
Redefining the major peanut allergens.Immunol Res. 2013 Mar;55(1-3):125-34. doi: 10.1007/s12026-012-8355-x. Immunol Res. 2013. PMID: 22948807 Free PMC article. Review.
-
[Progress of researches on the allergens Ara h 1, Ara h 2 and Ara h 3 from peanut].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010 Dec;27(6):1401-5. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010. PMID: 21375004 Review. Chinese.
Cited by
-
Effect of chemical modifications on allergenic potency of peanut proteins.Allergy Asthma Proc. 2015 May-Jun;36(3):185-91. doi: 10.2500/aap.2015.36.3840. Allergy Asthma Proc. 2015. PMID: 25976435 Free PMC article.
-
Ara h 2 is the dominant peanut allergen despite similarities with Ara h 6.J Allergy Clin Immunol. 2020 Sep;146(3):621-630.e5. doi: 10.1016/j.jaci.2020.03.026. Epub 2020 Apr 13. J Allergy Clin Immunol. 2020. PMID: 32298698 Free PMC article.
-
Development and evaluation of mouse anti-Ara h 1 and Ara h 3 IgE monoclonal antibodies for advancing peanut allergy research.MethodsX. 2023 Nov 4;11:102470. doi: 10.1016/j.mex.2023.102470. eCollection 2023 Dec. MethodsX. 2023. PMID: 38034322 Free PMC article.
-
Release of Major Peanut Allergens from Their Matrix under Various pH and Simulated Saliva Conditions-Ara h2 and Ara h6 Are Readily Bio-Accessible.Nutrients. 2018 Sep 11;10(9):1281. doi: 10.3390/nu10091281. Nutrients. 2018. PMID: 30208580 Free PMC article.
-
Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2.J Allergy Clin Immunol. 2017 Jan;139(1):366-369.e2. doi: 10.1016/j.jaci.2016.06.053. Epub 2016 Aug 20. J Allergy Clin Immunol. 2017. PMID: 27554819 Free PMC article. No abstract available.
References
-
- Burks AW. Peanut allergy. Lancet. 2008;371:1538–1546. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–125. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–1018. - PubMed
-
- Yunginger JW, Squillace DL, Jones RT, Helm RM. Fatal anaphylactic reactions induced by peanuts. Allergy Proc. 1989;10:249–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

