Siglecs as sensors of self in innate and adaptive immune responses
- PMID: 22288608
- PMCID: PMC3335958
- DOI: 10.1111/j.1749-6632.2011.06362.x
Siglecs as sensors of self in innate and adaptive immune responses
Abstract
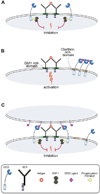
Siglecs are expressed on most white blood cells of the immune system and are known to modulate the activity of cell signaling receptors via regulatory motifs in their cytoplasmic domains. This immunoglobulin subfamily of coreceptors recognize sialic acid containing glycans as ligands, which are found on glycoproteins and glycolipids of all mammalian cells. By virtue of their ability to recognize this common structural element, siglecs are increasingly recognized for their ability to help immune cells distinguish between self and nonself, and dampen autoimmune responses.
© 2012 New York Academy of Sciences.
Figures


References
-
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. - PubMed
-
- Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36:1467–1471. - PubMed
-
- Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

