Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms
- PMID: 22289042
- PMCID: PMC3480182
- DOI: 10.1037/a0026708
Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms
Abstract
From its origins in how the brain controls the endocrine system via the hypothalamus and pituitary gland, neuroendocrinology has evolved into a science that now includes hormone action on many aspects of brain function. These actions involve the whole central nervous system and not just the hypothalamus. Advances in our understanding of cellular and molecular actions of steroid hormones have gone beyond the important cell nuclear actions of steroid hormone receptors to include signaling pathways that intersect with other mediators such as neurotransmitters and neuromodulators. This has, in turn, broadened the search for and identification of steroid receptors to include nonnuclear sites in synapses, dendrites, mitochondria, and glial cells, as well as cell nuclei. The study of estrogen receptors and estrogen actions on processes related to cognition, mood, autonomic regulation, pain, and neuroprotection, among other functions, has led the way in this new view of hormone actions on the brain. In this review, we summarize past and current work in our laboratory on this topic. This exciting and growing field involving many laboratories continues to reshape our ideas and approaches to neuroendocrinology both at the bench and the bedside.
Figures




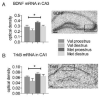
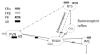

References
-
- Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:10–10. - PubMed
-
- Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, nonpeptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and L-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:245–52. - PubMed
-
- Becker JB. Direct effect of 17b-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990;5:157–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

