Heparin in pregnant women with previous placenta-mediated pregnancy complications: a prospective, randomized, multicenter, controlled clinical trial
- PMID: 22289887
- PMCID: PMC3321853
- DOI: 10.1182/blood-2011-11-391383
Heparin in pregnant women with previous placenta-mediated pregnancy complications: a prospective, randomized, multicenter, controlled clinical trial
Abstract
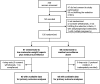
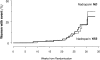
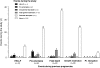
To assess whether antithrombotic prophylaxis with low-molecular-weight heparin effectively prevents recurrence of late pregnancy complications, 135 women with previous history of preeclampsia, hemolytic anemia, elevated liver enzymes and low platelet count syndrome, intrauterine fetal death, fetal growth restriction, or placental abruption who had been referred within the 12th gestational week were randomized to medical surveillance alone (n = 68) or combined to open-label nadroparin (3800 IU daily subcutaneous injections) treatment (n = 67) in the setting of a randomized, parallel-group, superiority trial, run in Italy from April 2007 to April 2010. Primary outcome was a composite end point of late-pregnancy complications. Analysis was by intention to treat. The study was stopped for futility at the time of the first planned interim analysis. Among the 128 women eventually available for final analyses, 13 of the 63 (21%) randomized to nadroparin compared with 12 of the 65 (18%) on medical surveillance alone progressed to the primary end point. The absolute event risk difference between treatment arms (2.2; -1.6 to 16.0) was not statistically significant (P = .76). Thus, nadroparin did not prevent late-pregnancy complications in women at risk of recurrence. This finding challenges the role of antithrombotic prophylaxis with low-molecular-weight heparin in the prevention of recurrent late pregnancy complications The trial was registered at http://ricerca-clinica.agenziafarmaco.it as EudraCT 2006-004205-26.
Figures
Comment in
-
Evidence over hope for pregnancy complications.Blood. 2012 Apr 5;119(14):3192-3. doi: 10.1182/blood-2012-02-410365. Blood. 2012. PMID: 22493211
References
-
- Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191(2):412–424. - PubMed
-
- Rodger MA, Paidas M, McLintock C, et al. Inherited thrombophilia and pregnancy complications revisited. Obstet Gynecol. 2008;112(2):320–324. - PubMed
-
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. - PubMed
-
- Kok JH, Den Ouden AL, Verloove-Vanhoric SP, Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol. 1998;105(2):162–168. - PubMed
-
- Viero S, Chadda V, Alkazaleh F, et al. Prognostic value of placental ultrasound in pregnancies complicated by absent end-diastolic flow velocity in the umbilical arteries. Placenta. 2004;25(8):735–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

