Immunotherapy for the treatment of glioblastoma
- PMID: 22290259
- PMCID: PMC3269657
- DOI: 10.1097/PPO.0b013e3182431a73
Immunotherapy for the treatment of glioblastoma
Abstract
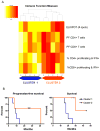
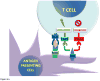
Glioblastoma, the most aggressive primary brain tumor, thrives in a microenvironment of relative immunosuppression within the relatively immune-privileged central nervous system. Despite treatments with surgery, radiation therapy, and chemotherapy, prognosis remains poor. The recent success of immunotherapy in the treatment of other cancers has renewed interest in vaccine therapy for the treatment of gliomas. In this article, we outline various immunotherapeutic strategies, review recent clinical trials data, and discuss the future of vaccine therapy for glioblastoma.
Figures





References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. - PubMed
-
- Abbas . Diseases of Immunity. In: Fausto KA, editor. Robbins and Cotran Pathologic Basis of Disease. 7. Philadelphia: Elsevier Saunders; 2005. pp. 193–267.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

