Hazard ratios in cancer clinical trials--a primer
- PMID: 22290283
- PMCID: PMC7457144
- DOI: 10.1038/nrclinonc.2011.217
Hazard ratios in cancer clinical trials--a primer
Abstract
The increase and diversity of clinical trial data has resulted in a greater reliance on statistical analyses to discern value. Assessing differences between two similar survival curves can pose a challenge for those without formal training in statistical interpretation; therefore, there has been an increased reliance on hazard ratios often to the exclusion of more-traditional survival measures. However, because a hazard ratio lacks dimensions it can only inform the reader about the reliability and uniformity of the data. It does not provide practitioners with quantitative values they can use, nor does it provide information they can discuss with patients. Motivated by a non-scientific poll of oncologists in training and those with board certification that suggested only a limited understanding of the derivation of hazard ratios we undertook this presentation of hazard ratios: a measure of treatment efficacy that is increasingly used and often misused.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


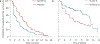
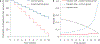
References
-
- Kaplan EL & Meier P Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc 53, 457–481 (1958).
-
- Escudier B et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med 356, 125–134 (2007). - PubMed
-
- Motzer RJ et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med 356, 115–124 (2007). - PubMed
-
- Armitage P, Berry G & Matthews JNS Statistical Methods in Medical Research, 4th edn, 568–590 (Blackwell Science Ltd, Oxford, 2002).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

