MicroRNA control of bone formation and homeostasis
- PMID: 22290358
- PMCID: PMC3589914
- DOI: 10.1038/nrendo.2011.234
MicroRNA control of bone formation and homeostasis
Abstract
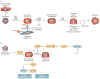
MicroRNAs (miRNAs) repress cellular protein levels to provide a sophisticated parameter of gene regulation that coordinates a broad spectrum of biological processes. Bone organogenesis is a complex process involving the differentiation and crosstalk of multiple cell types for formation and remodeling of the skeleton. Inhibition of mRNA translation by miRNAs has emerged as an important regulator of developmental osteogenic signaling pathways, osteoblast growth and differentiation, osteoclast-mediated bone resorption activity and bone homeostasis in the adult skeleton. miRNAs control multiple layers of gene regulation for bone development and postnatal functions, from the initial response of stem/progenitor cells to the structural and metabolic activity of the mature tissue. This Review brings into focus an emerging concept of bone-regulating miRNAs, the evidence for which has been gathered largely from in vivo mouse models and in vitro studies in human and mouse skeletal cell populations. Characterization of miRNAs that operate through tissue-specific transcription factors in osteoblast and osteoclast lineage cells, as well as intricate feedforward and reverse loops, has provided novel insights into the supervision of signaling pathways and regulatory networks controlling normal bone formation and turnover. The current knowledge of miRNAs characteristic of human pathologic disorders of the skeleton is presented with a future goal towards translational studies.
Conflict of interest statement
The authors declare no competing interests.
Figures

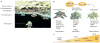




References
-
- Del Fattore A, Teti A, Rucci N. Osteoclast receptors and signaling. Arch Biochem Biophys. 2008;473:147–160. - PubMed
-
- Ross FP, Teitelbaum SL. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. - PubMed
-
- Miyazaki T, Tanaka S, Sanjay A, Baron R. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. - PubMed
-
- Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

