A novel role for copper in Ras/mitogen-activated protein kinase signaling
- PMID: 22290441
- PMCID: PMC3302449
- DOI: 10.1128/MCB.05722-11
A novel role for copper in Ras/mitogen-activated protein kinase signaling
Abstract
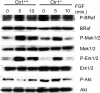
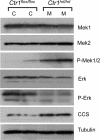
Copper (Cu) is essential for development and proliferation, yet the cellular requirements for Cu in these processes are not well defined. We report that Cu plays an unanticipated role in the mitogen-activated protein (MAP) kinase pathway. Ablation of the Ctr1 high-affinity Cu transporter in flies and mouse cells, mutation of Ctr1, and Cu chelators all reduce the ability of the MAP kinase kinase Mek1 to phosphorylate the MAP kinase Erk. Moreover, mice bearing a cardiac-tissue-specific knockout of Ctr1 are deficient in Erk phosphorylation in cardiac tissue. in vitro investigations reveal that recombinant Mek1 binds two Cu atoms with high affinity and that Cu enhances Mek1 phosphorylation of Erk in a dose-dependent fashion. Coimmunoprecipitation experiments suggest that Cu is important for promoting the Mek1-Erk physical interaction that precedes the phosphorylation of Erk by Mek1. These results demonstrate a role for Ctr1 and Cu in activating a pathway well known to play a key role in normal physiology and in cancer.
Figures







References
-
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. 2008. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum. Mutat. 29:992–1006 - PubMed
-
- Baker NE, Rubin GM. 1992. Ellipse mutations in the Drosophila homologue of the EGF receptor affect pattern formation, cell division, and cell death in eye imaginal discs. Dev. Biol. 150:381–396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
