SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells
- PMID: 22290867
- PMCID: PMC6993144
- DOI: 10.1002/stem.1049
SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells
Abstract
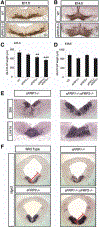
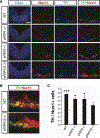
Secreted Frizzled related proteins (sFRPs) are a family of proteins that modulate Wnt signaling, which in turn regulates multiple aspects of ventral midbrain (VM) and dopamine (DA) neuron development. However, it is not known which Wnt signaling branch and what aspects of midbrain DA neuron development are regulated by sFRPs. Here, we show that sFRP1 and sFRP2 activate the Wnt/planar-cell-polarity/Rac1 pathway in DA cells. In the developing VM, sFRP1 and sFRP2 are expressed at low levels, and sFRP1-/- or sFRP2-/- mice had no detectable phenotype. However, compound sFRP1-/-;sFRP2-/- mutants revealed a Wnt/PCP phenotype similar to that previously described for Wnt5a-/- mice. This included an anteroposterior shortening of the VM, a lateral expansion of the Shh domain and DA lineage markers (Lmx1a and Th), as well as an accumulation of Nurr1+ precursors in the VM. In vitro experiments showed that, while very high concentrations of SFRP1 had a negative effect on cell survival, low/medium concentrations of sFRP1 or sFRP2 promoted the DA differentiation of progenitors derived from primary VM cultures or mouse embryonic stem cells (ESCs), mimicking the effects of Wnt5a. We thus conclude that the main function of sFRP1 and sFRP2 is to enhance Wnt/PCP signaling in DA cells and to regulate Wnt/PCP-dependent functions in midbrain development. Moreover, we suggest that low-medium concentrations of sFRPs may be used to enhance the DA differentiation of ESCs and improve their therapeutic application.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Figures




References
-
- Uren A, Reichsman F, Anest V et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 2000;275:4374–4382. - PubMed
-
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development 2009;136:3205–3214. - PubMed
-
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat Rev Neurosci 2005;6: 351–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

