4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) is a fluorescent substrate for the human serotonin transporter
- PMID: 22291010
- PMCID: PMC3308769
- DOI: 10.1074/jbc.M111.267757
4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) is a fluorescent substrate for the human serotonin transporter
Abstract
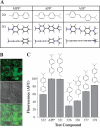
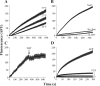
Monoamine transporters terminate synaptic neurotransmission and are molecular targets for antidepressants and psychostimulants. Fluorescent reporters can monitor real-time transport and are amenable for high-throughput screening. However, until now, their use has mostly been successful to study the catecholamine transporters but not the serotonin (5HT) transporter. Here, we use fluorescence microscopy, electrophysiology, pharmacology, and molecular modeling to compare fluorescent analogs of 1-methyl-4-phenylpyridinium (MPP(+)) as reporters for the human serotonin transporter (hSERT) in single cells. The fluorescent substrate 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP(+)) exhibits superior fluorescence uptake in hSERT-expressing HEK293 cells than other MPP(+) analogs tested. APP(+) uptake is Na(+)- and Cl(-)-dependent, displaced by 5HT, and inhibited by fluoxetine, suggesting APP(+) specifically monitors hSERT activity. ASP(+), which was previously used to study catecholamine transporters, is 10 times less potent than APP(+) at inhibiting 5HT uptake and has minimal hSERT-mediated uptake. Furthermore, in hSERT-expressing oocytes voltage-clamped to -60 mV, APP(+) induced fluoxetine-sensitive hSERT-mediated inward currents, indicating APP(+) is a substrate, whereas ASP(+) induced hSERT-mediated outward currents and counteracted 5HT-induced hSERT currents, indicating ASP(+) possesses activity as an inhibitor. Extra-precise ligand receptor docking of APP(+) and ASP(+) in an hSERT homology model showed both ASP(+) and APP(+) docked favorably within the active region; accordingly, comparable concentrations are required to elicit their opposite electrophysiological responses. We conclude APP(+) is better suited than ASP(+) to study hSERT transport fluorometrically.
Figures
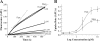




Similar articles
-
APP+, a fluorescent analogue of the neurotoxin MPP+, is a marker of catecholamine neurons in brain tissue, but not a fluorescent false neurotransmitter.ACS Chem Neurosci. 2013 May 15;4(5):858-69. doi: 10.1021/cn400038u. Epub 2013 May 6. ACS Chem Neurosci. 2013. PMID: 23647019 Free PMC article.
-
Binding-induced fluorescence of serotonin transporter ligands: A spectroscopic and structural study of 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP(+)) and APP(+) analogues.ACS Chem Neurosci. 2014 Apr 16;5(4):296-304. doi: 10.1021/cn400230x. Epub 2014 Feb 5. ACS Chem Neurosci. 2014. PMID: 24460204 Free PMC article.
-
Handling of intracellular K+ determines voltage dependence of plasmalemmal monoamine transporter function.Elife. 2021 Jun 1;10:e67996. doi: 10.7554/eLife.67996. Elife. 2021. PMID: 34061030 Free PMC article.
-
Y95 and E444 interaction required for high-affinity S-citalopram binding in the human serotonin transporter.ACS Chem Neurosci. 2011 Feb 16;2(2):75-81. doi: 10.1021/cn100066p. Epub 2010 Oct 27. ACS Chem Neurosci. 2011. PMID: 22778858 Free PMC article. Review.
-
Impact of oligomerization on the function of the human serotonin transporter.Biochem Soc Trans. 2001 Nov;29(Pt 6):732-6. doi: 10.1042/0300-5127:0290732. Biochem Soc Trans. 2001. PMID: 11709065 Review.
Cited by
-
Functional characterization of N-octyl 4-methylamphetamine variants and related bivalent compounds at the dopamine and serotonin transporters using Ca2+ channels as sensors.Toxicol Appl Pharmacol. 2021 May 15;419:115513. doi: 10.1016/j.taap.2021.115513. Epub 2021 Mar 27. Toxicol Appl Pharmacol. 2021. PMID: 33785354 Free PMC article.
-
Dissociable effects of the prodrug phendimetrazine and its metabolite phenmetrazine at dopamine transporters.Sci Rep. 2016 Aug 12;6:31385. doi: 10.1038/srep31385. Sci Rep. 2016. PMID: 27514281 Free PMC article.
-
Electrical coupling between the human serotonin transporter and voltage-gated Ca(2+) channels.Cell Calcium. 2014 Jul;56(1):25-33. doi: 10.1016/j.ceca.2014.04.003. Epub 2014 Apr 27. Cell Calcium. 2014. PMID: 24854234 Free PMC article.
-
Novel serotonin transporter regulators: Natural aristolane- and nardosinane- types of sesquiterpenoids from Nardostachys chinensis Batal.Sci Rep. 2017 Nov 8;7(1):15114. doi: 10.1038/s41598-017-15483-6. Sci Rep. 2017. PMID: 29118341 Free PMC article.
-
Molecular docking and biochemical validation of (-)-syringaresinol-4-O-β-D-apiofuranosyl-(1→2)-β-D-glucopyranoside binding to an allosteric site in monoamine transporters.Front Pharmacol. 2022 Oct 28;13:1018473. doi: 10.3389/fphar.2022.1018473. eCollection 2022. Front Pharmacol. 2022. PMID: 36386236 Free PMC article.
References
-
- Schloss P., Williams D. C. (1998) The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 12, 115–121 - PubMed
-
- Stahl S. M. (1998) Mechanism of action of serotonin-selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect Disord. 51, 215–235 - PubMed
-
- Murphy D. L., Lerner A., Rudnick G., Lesch K. P. (2004) Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Mol. Interv. 4, 109–123 - PubMed
-
- Feighner J. P. (1994) Clinical effects of serotonin reuptake inhibitors–a review. Fortschr. Neurol. Psychiatr. 62, 9–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

