Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury
- PMID: 22291096
- PMCID: PMC3280874
- DOI: 10.1084/jem.20111493
Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury
Abstract
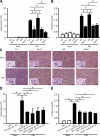
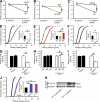
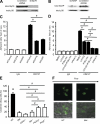
Neutrophils trigger inflammation-induced acute kidney injury (AKI), a frequent and potentially lethal occurrence in humans. Molecular mechanisms underlying neutrophil recruitment to sites of inflammation have proved elusive. In this study, we demonstrate that SLP-76 (SH2 domain-containing leukocyte phosphoprotein of 76 kD) and ADAP (adhesion and degranulation promoting adaptor protein) are involved in E-selectin-mediated integrin activation and slow leukocyte rolling, which promotes ischemia-reperfusion-induced AKI in mice. By using genetically engineered mice and transduced Slp76(-/-) primary leukocytes, we demonstrate that ADAP as well as two N-terminal-located tyrosines and the SH2 domain of SLP-76 are required for downstream signaling and slow leukocyte rolling. The Tec family kinase Bruton tyrosine kinase is downstream of SLP-76 and, together with ADAP, regulates PI3Kγ (phosphoinositide 3-kinase-γ)- and PLCγ2 (phospholipase Cγ2)-dependent pathways. Blocking both pathways completely abolishes integrin affinity and avidity regulation. Thus, SLP-76 and ADAP are involved in E-selectin-mediated integrin activation and neutrophil recruitment to inflamed kidneys, which may underlie the development of life-threatening ischemia-reperfusion-induced AKI in humans.
Figures

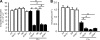
References
-
- Altavilla D., Squadrito F., Ioculano M., Canale P., Campo G.M., Zingarelli B., Caputi A.P. 1994. E-selectin in the pathogenesis of experimental myocardial ischemia-reperfusion injury. Eur. J. Pharmacol. 270:45–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

