Apical Ca2+-activated potassium channels in mouse parotid acinar cells
- PMID: 22291145
- PMCID: PMC3269790
- DOI: 10.1085/jgp.201110718
Apical Ca2+-activated potassium channels in mouse parotid acinar cells
Abstract
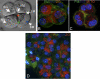
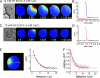
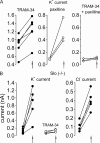
Ca(2+) activation of Cl and K channels is a key event underlying stimulated fluid secretion from parotid salivary glands. Cl channels are exclusively present on the apical plasma membrane (PM), whereas the localization of K channels has not been established. Mathematical models have suggested that localization of some K channels to the apical PM is optimum for fluid secretion. A combination of whole cell electrophysiology and temporally resolved digital imaging with local manipulation of intracellular [Ca(2+)] was used to investigate if Ca(2+)-activated K channels are present in the apical PM of parotid acinar cells. Initial experiments established Ca(2+)-buffering conditions that produced brief, localized increases in [Ca(2+)] after focal laser photolysis of caged Ca(2+). Conditions were used to isolate K(+) and Cl(-) conductances. Photolysis at the apical PM resulted in a robust increase in K(+) and Cl(-) currents. A localized reduction in [Ca(2+)] at the apical PM after photolysis of Diazo-2, a caged Ca(2+) chelator, resulted in a decrease in both K(+) and Cl(-) currents. The K(+) currents evoked by apical photolysis were partially blocked by both paxilline and TRAM-34, specific blockers of large-conductance "maxi-K" (BK) and intermediate K (IK), respectively, and almost abolished by incubation with both antagonists. Apical TRAM-34-sensitive K(+) currents were also observed in BK-null parotid acini. In contrast, when the [Ca(2+)] was increased at the basal or lateral PM, no increase in either K(+) or Cl(-) currents was evoked. These data provide strong evidence that K and Cl channels are similarly distributed in the apical PM. Furthermore, both IK and BK channels are present in this domain, and the density of these channels appears higher in the apical versus basolateral PM. Collectively, this study provides support for a model in which fluid secretion is optimized after expression of K channels specifically in the apical PM.
Figures




References
-
- Cook D.I., Van Lennep E.W., Roberts M.L., Young J.A. 1994. Secretion by the major salivary glands. In Physiology of the Gastrointestinal Tract. Third edition. Raven Press, New York. 1061–1117.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

