The importance of adjuvant formulation in the development of a tuberculosis vaccine
- PMID: 22291184
- PMCID: PMC3288309
- DOI: 10.4049/jimmunol.1102696
The importance of adjuvant formulation in the development of a tuberculosis vaccine
Abstract
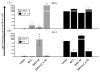
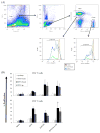
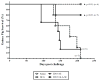
An effective protein-based vaccine for tuberculosis will require a safe and effective adjuvant. There are few adjuvants in approved human vaccines, including alum and the oil-in-water-based emulsions MF59 (Novartis Vaccines and Diagnostics), AS03 and AS04 (GlaxoSmithKline Biologics), AF03 (Sanofi), and liposomes (Crucell). When used with pure, defined proteins, both alum and emulsion adjuvants are effective at inducing primarily humoral responses. One of the newest adjuvants in approved products is AS04, which combines monophosphoryl lipid A, a TLR-4 agonist, with alum. In this study, we compared two adjuvants: a stable oil-in-water emulsion (SE) and a stable oil-in-water emulsion incorporating glucopyranosyl lipid adjuvant, a synthetic TLR-4 agonist (GLA-SE), each together with a recombinant protein, ID93. Both the emulsion SE and GLA-SE adjuvants induce potent cellular responses in combination with ID93 in mice. ID93/SE induced Th2-biased immune responses, whereas ID93/GLA-SE induced multifunctional CD4(+) Th1 cell responses (IFN-γ, TNF-α, and IL-2). The ID93/GLA-SE vaccine candidate induced significant protection in mice and guinea pigs, whereas no protection was observed with ID93/SE, as assessed by reductions in bacterial burden, survival, and pathology. These results highlight the importance of properly formulating subunit vaccines with effective adjuvants for use against tuberculosis.
Conflict of interest statement
Dr. Reed is a founder of, and holds an equity interest in, Immune Design Corp., a licensee of certain rights associated with GLA.
Figures
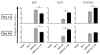


References
-
- World Health Organization. Global Tuberculosis Control. 2010. p. 2010.
-
- Vogel FR, Caillet C, Kusters IC, Haensler J. Emulsion-based adjuvants for influenza vaccines. Expert Rev Vaccines. 2009;8:483–492. - PubMed
-
- Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

