Randomized, double-blind, placebo-controlled, linear dose, crossover study to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects
- PMID: 22291473
- PMCID: PMC3267522
- DOI: 10.2147/DMSO.S27665
Randomized, double-blind, placebo-controlled, linear dose, crossover study to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects
Retraction in
-
Randomized, double-blind, placebo-controlled, linear dose, crossover study to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects [Retraction].Diabetes Metab Syndr Obes. 2014 Oct 16;7:467. doi: 10.2147/DMSO.S75357. eCollection 2014. Diabetes Metab Syndr Obes. 2014. PMID: 25340633 Free PMC article. No abstract available.
Abstract
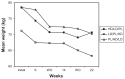
Background: Adult weight gain and obesity have become worldwide problems. Issues of cost and potential side effects of prescription weight loss drugs have led overweight and obese adults to try nutraceuticals that may aid weight loss. One promising nutraceutical is green coffee extract, which contains high concentrations of chlorogenic acids that are known to have health benefits and to influence glucose and fat metabolism. A 22-week crossover study was conducted to examine the efficacy and safety of a commercial green coffee extract product GCA™ at reducing weight and body mass in 16 overweight adults.
Methods: Subjects received high-dose GCA (1050 mg), low-dose GCA (700 mg), or placebo in separate six-week treatment periods followed by two-week washout periods to reduce any influence of preceding treatment. Treatments were counterbalanced between subjects. Primary measurements were body weight, body mass index, and percent body fat. Heart rate and blood pressure were also measured.
Results: Significant reductions were observed in body weight (-8.04 ± 2.31 kg), body mass index (-2.92 ± 0.85 kg/m(2)), and percent body fat (-4.44% ± 2.00%), as well as a small decrease in heart rate (-2.56 ± 2.85 beats per minute), but with no significant changes to diet over the course of the study. Importantly, the decreases occurred when subjects were taking GCA. Body mass index for six subjects shifted from preobesity to the normal weight range (<25.00 kg/m(2)).
Conclusion: The results are consistent with human and animal studies and a meta-analysis of the efficacy of green coffee extract in weight loss. The results suggest that GCA may be an effective nutraceutical in reducing weight in preobese adults, and may be an inexpensive means of preventing obesity in overweight adults.
Keywords: blood pressure; body fat mass; body mass index; chlorogenic acid; green coffee bean extract; heart rate; weight loss.
Figures
Comment in
-
If it seems too good to be true..Am Fam Physician. 2015 May 15;91(10):729-30. Am Fam Physician. 2015. PMID: 25978205 No abstract available.
References
-
- World Health Organization (WHO) Fact Sheet 311. Geneva: WHO; 2011.
-
- Market Research News. Global market for weight loss worth US$586.3 billion by 2014. [Accessed November 18, 2011]. Available from: http://www.salisonline.org/market-research/global-market-for-weight-loss...
-
- Vinson JA, Al Kharrat H, Shuta D. Investigation of an amylase inhibitor on human glucose absorption after starch consumption. Open Nutraceuticals J. 2009;2:88–91.
-
- Lopez-Garcia E, van Dam RM, Rajpathak S, Willett WC, Manson JE, Hu FB. Changes in caffeine intake and long-term weight change in men and women. Am J Clin Nutr. 2006;83:674–680. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources