Miniaturized pH Sensors Based on Zinc Oxide Nanotubes/Nanorods
- PMID: 22291545
- PMCID: PMC3260622
- DOI: 10.3390/s91108911
Miniaturized pH Sensors Based on Zinc Oxide Nanotubes/Nanorods
Abstract
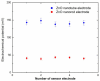
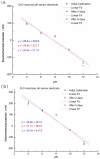
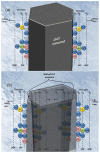
ZnO nanotubes and nanorods grown on gold thin film were used to create pH sensor devices. The developed ZnO nanotube and nanorod pH sensors display good reproducibility, repeatability and long-term stability and exhibit a pH-dependent electrochemical potential difference versus an Ag/AgCl reference electrode over a large dynamic pH range. We found the ZnO nanotubes provide sensitivity as high as twice that of the ZnO nanorods, which can be ascribed to the fact that small dimensional ZnO nanotubes have a higher level of surface and subsurface oxygen vacancies and provide a larger effective surface area with higher surface-to-volume ratio as compared to ZnO nanorods, thus affording the ZnO nanotube pH sensor a higher sensitivity. Experimental results indicate ZnO nanotubes can be used in pH sensor applications with improved performance. Moreover, the ZnO nanotube arrays may find potential application as a novel material for measurements of intracellular biochemical species within single living cells.
Keywords: ZnO nanorods; ZnO nanotubes; pH sensors; potentiometric measurements.
Figures




References
-
- Wadeasa A., Nur O., Willander M. The effect of the interlayer design on the electroluminescence and electrical properties of n-ZnO nanorod/p-type blended polymer hybrid light emitting diodes. Nanotechnology. 2009;20:065710. - PubMed
-
- Willander M., Lozovik Y.E., Zhao Q.X., Nur O., Hu Q.H., Klason P. Excitonic effects in ZnO nanowires and hollow nanotubes. Proc. SPIE. 2007;6486:648614.
-
- Riaz M., Fulati A., Zhao Q.X., Nur O., Willander M., Klason P. Buckling and mechanical instability of ZnO nanorods grown on different substrates under uniaxial compression. Nanotechnology. 2008;19:415708. - PubMed
-
- Riaz M., Fulati A., Yang L.L., Nur O., Willander M., Klason P. Bending flexibility, kinking, and buckling characterization of ZnO nanorods/nanowires grown on different substrates by high and low temperature methods. J. Appl. Phys. 2008;104:104306.
-
- Liu J.P., Guo C.X., Li C.M., Li Y.Y., Chi Q.B., Huang X.T., Liao L., Yu T. Carbon-decorated ZnO nanowire array: a novel platform for direct electrochemistry of enzymes and biosensing applications. Electrochem. Commun. 2009;11:202–205.
LinkOut - more resources
Full Text Sources
Other Literature Sources

