The bacterial cytoskeleton modulates motility, type 3 secretion, and colonization in Salmonella
- PMID: 22291596
- PMCID: PMC3266929
- DOI: 10.1371/journal.ppat.1002500
The bacterial cytoskeleton modulates motility, type 3 secretion, and colonization in Salmonella
Abstract
Although there have been great advances in our understanding of the bacterial cytoskeleton, major gaps remain in our knowledge of its importance to virulence. In this study we have explored the contribution of the bacterial cytoskeleton to the ability of Salmonella to express and assemble virulence factors and cause disease. The bacterial actin-like protein MreB polymerises into helical filaments and interacts with other cytoskeletal elements including MreC to control cell-shape. As mreB appears to be an essential gene, we have constructed a viable ΔmreC depletion mutant in Salmonella. Using a broad range of independent biochemical, fluorescence and phenotypic screens we provide evidence that the Salmonella pathogenicity island-1 type three secretion system (SPI1-T3SS) and flagella systems are down-regulated in the absence of MreC. In contrast the SPI-2 T3SS appears to remain functional. The phenotypes have been further validated using a chemical genetic approach to disrupt the functionality of MreB. Although the fitness of ΔmreC is reduced in vivo, we observed that this defect does not completely abrogate the ability of Salmonella to cause disease systemically. By forcing on expression of flagella and SPI-1 T3SS in trans with the master regulators FlhDC and HilA, it is clear that the cytoskeleton is dispensable for the assembly of these structures but essential for their expression. As two-component systems are involved in sensing and adapting to environmental and cell surface signals, we have constructed and screened a panel of such mutants and identified the sensor kinase RcsC as a key phenotypic regulator in ΔmreC. Further genetic analysis revealed the importance of the Rcs two-component system in modulating the expression of these virulence factors. Collectively, these results suggest that expression of virulence genes might be directly coordinated with cytoskeletal integrity, and this regulation is mediated by the two-component system sensor kinase RcsC.
Conflict of interest statement
The authors have declared that no competing interests exist.
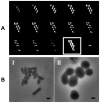
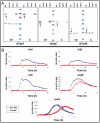
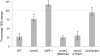
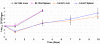
Figures



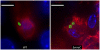
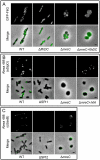
References
-
- Pang T, Levine MM, Ivanoff B, Wain J, Finlay BB. Typhoid fever-important issues still remain. Trends Microbiol. 1998;6:131–133. - PubMed
-
- Lilic M, Galkin VE, Orlova A, VanLoock MS, Egelman EH, et al. Salmonella SipA polymerizes actin by stapling filaments with nonglobular protein arms. Science. 2003;301:1918–1921. - PubMed
-
- Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4:629–636. - PubMed
-
- Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: a global problem. J Med Microbiol. 1996;44:317–319. - PubMed
-
- Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

