Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity
- PMID: 22291601
- PMCID: PMC3266875
- DOI: 10.1371/journal.pgen.1002448
Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity
Abstract
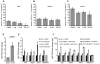
Recent studies have revealed an important role for hormones in plant immunity. We are now beginning to understand the contribution of crosstalk among different hormone signaling networks to the outcome of plant-pathogen interactions. Cytokinins are plant hormones that regulate development and responses to the environment. Cytokinin signaling involves a phosphorelay circuitry similar to two-component systems used by bacteria and fungi to perceive and react to various environmental stimuli. In this study, we asked whether cytokinin and components of cytokinin signaling contribute to plant immunity. We demonstrate that cytokinin levels in Arabidopsis are important in determining the amplitude of immune responses, ultimately influencing the outcome of plant-pathogen interactions. We show that high concentrations of cytokinin lead to increased defense responses to a virulent oomycete pathogen, through a process that is dependent on salicylic acid (SA) accumulation and activation of defense gene expression. Surprisingly, treatment with lower concentrations of cytokinin results in increased susceptibility. These functions for cytokinin in plant immunity require a host phosphorelay system and are mediated in part by type-A response regulators, which act as negative regulators of basal and pathogen-induced SA-dependent gene expression. Our results support a model in which cytokinin up-regulates plant immunity via an elevation of SA-dependent defense responses and in which SA in turn feedback-inhibits cytokinin signaling. The crosstalk between cytokinin and SA signaling networks may help plants fine-tune defense responses against pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





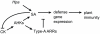
References
-
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Feys BJ, Parker JE. Interplay of signaling pathways in plant disease resistance. Trends in Genetics. 2000;16:449–455. - PubMed
-
- Spoel SH, Dong XN. Making sense of hormone crosstalk during plant immune responses. Cell Host & Microbe. 2008;3:348–351. - PubMed
-
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD. Pathological hormone imbalances. Current Opinion in Plant Biology. 2007;10:372–379. - PubMed
-
- Argueso CT, Ferreira FJ, Kieber JJ. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell and Environment. 2009;32:1147–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

