A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs
- PMID: 22291913
- PMCID: PMC3265466
- DOI: 10.1371/journal.pone.0030176
A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs
Abstract
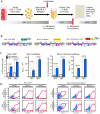
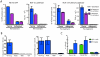
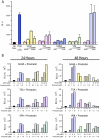
Latently infected cells form the major obstacle to HIV eradication. Studies of HIV latency have been generally hindered by the lack of a robust and rapidly deployable cell model that involves primary human CD4 T lymphocytes. Latently infected cell lines have proven useful, but it is unclear how closely these proliferating cells recapitulate the conditions of viral latency in non-dividing CD4 T lymphocytes in vivo. Current primary lymphocyte models more closely reflect the in vivo state of HIV latency, but they are limited by protracted culture periods and often low cell yields. Additionally, these models are always established in a single latently infected cell type that may not reflect the heterogeneous nature of the latent reservoir. Here we describe a rapid, sensitive, and quantitative primary cell model of HIV-1 latency with replication competent proviruses and multiple reporters to enhance the flexibility of the system. In this model, post-integration HIV-1 latency can be established in all populations of CD4 T cells, and reactivation of latent provirus assessed within 7 days. The kinetics and magnitude of reactivation were evaluated after stimulation with various cytokines, small molecules, and T-cell receptor agonists. Reactivation of latent HIV proviruses was readily detected in the presence of strong activators of NF-κB. Latently infected transitional memory CD4 T cells proved more responsive to these T-cell activators than latently infected central memory cells. These findings reveal potentially important biological differences within the latently infected pool of memory CD4 T cells and describe a flexible primary CD4 T-cell system to evaluate novel antagonists of HIV latency.
Conflict of interest statement
Figures


References
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
-
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

