Biochemical discrimination between selenium and sulfur 2: mechanistic investigation of the selenium specificity of human selenocysteine lyase
- PMID: 22291978
- PMCID: PMC3266904
- DOI: 10.1371/journal.pone.0030528
Biochemical discrimination between selenium and sulfur 2: mechanistic investigation of the selenium specificity of human selenocysteine lyase
Abstract
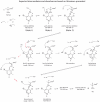
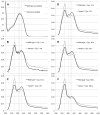
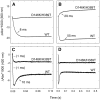
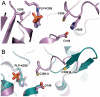
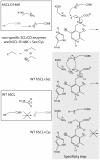
Selenium is an essential trace element incorporated into selenoproteins as selenocysteine. Selenocysteine (Sec) lyases (SCLs) and cysteine (Cys) desulfurases (CDs) catalyze the removal of selenium or sulfur from Sec or Cys, respectively, and generally accept both substrates. Intriguingly, human SCL (hSCL) is specific for Sec even though the only difference between Sec and Cys is a single chalcogen atom.The crystal structure of hSCL was recently determined and gain-of-function protein variants that also could accept Cys as substrate were identified. To obtain mechanistic insight into the chemical basis for its substrate discrimination, we here report time-resolved spectroscopic studies comparing the reactions of the Sec-specific wild-type hSCL and the gain-of-function D146K/H389T variant, when given Cys as a substrate. The data are interpreted in light of other studies of SCL/CD enzymes and offer mechanistic insight into the function of the wild-type enzyme. Based on these results and previously available data we propose a reaction mechanism whereby the Sec over Cys specificity is achieved using a combination of chemical and physico-mechanical control mechanisms.
Conflict of interest statement
Figures
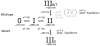
References
-
- Allmang C, Wurth L, Krol A. The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta. 2009;1790:1415–1423. - PubMed
-
- Yoshizawa S, Bock A. The many levels of control on bacterial selenoprotein synthesis. Biochim Biophys Acta. 2009;1790:1404–1414. - PubMed
-
- Letavayova L, Vlckova V, Brozmanova J. Selenium: from cancer prevention to DNA damage. Toxicology. 2006;227:1–14. - PubMed
-
- Angstwurm MW, Gaertner R. Practicalities of selenium supplementation in critically ill patients. Curr Opin Clin Nutr Metab Care. 2006;9:233–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

