Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds
- PMID: 22292013
- PMCID: PMC3266908
- DOI: 10.1371/journal.pone.0030662
Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds
Abstract
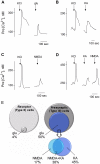
Recent studies suggest that l-glutamate may be an efferent transmitter released from axons innervating taste buds. In this report, we determined the types of ionotropic synaptic glutamate receptors present on taste cells and that underlie this postulated efferent transmission. We also studied what effect glutamate exerts on taste bud function. We isolated mouse taste buds and taste cells, conducted functional imaging using Fura 2, and used cellular biosensors to monitor taste-evoked transmitter release. The findings show that a large fraction of Presynaptic (Type III) taste bud cells (∼50%) respond to 100 µM glutamate, NMDA, or kainic acid (KA) with an increase in intracellular Ca(2+). In contrast, Receptor (Type II) taste cells rarely (4%) responded to 100 µM glutamate. At this concentration and with these compounds, these agonists activate glutamatergic synaptic receptors, not glutamate taste (umami) receptors. Moreover, applying glutamate, NMDA, or KA caused taste buds to secrete 5-HT, a Presynaptic taste cell transmitter, but not ATP, a Receptor cell transmitter. Indeed, glutamate-evoked 5-HT release inhibited taste-evoked ATP secretion. The findings are consistent with a role for glutamate in taste buds as an inhibitory efferent transmitter that acts via ionotropic synaptic glutamate receptors.
Conflict of interest statement
Figures


References
-
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. - PubMed
-
- Lu SG, Zhao FL, Herness S. Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett. 2003;351:157–160. - PubMed
-
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–658. - PubMed
-
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

