Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells
- PMID: 22292015
- PMCID: PMC3264594
- DOI: 10.1371/journal.pone.0030674
Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells
Abstract
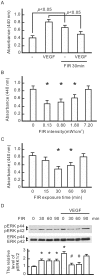
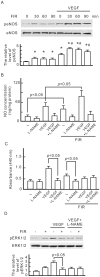
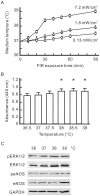
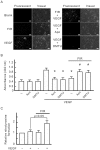
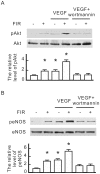
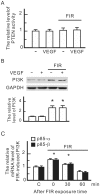
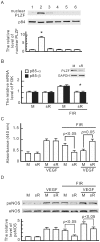
Many studies suggest that far-infrared (FIR) therapy can reduce the frequency of some vascular-related diseases. The non-thermal effect of FIR was recently found to play a role in the long-term protective effect on vascular function, but its molecular mechanism is still unknown. In the present study, we evaluated the biological effect of FIR on vascular endothelial growth factor (VEGF)-induced proliferation in human umbilical vein endothelial cells (HUVECs). We found that FIR ranging 3∼10 µm significantly inhibited VEGF-induced proliferation in HUVECs. According to intensity and time course analyses, the inhibitory effect of FIR peaked at an effective intensity of 0.13 mW/cm(2) at 30 min. On the other hand, a thermal effect did not inhibit VEGF-induced proliferation in HUVECs. FIR exposure also inhibited the VEGF-induced phosphorylation of extracellular signal-regulated kinases in HUVECs. FIR exposure further induced the phosphorylation of endothelial nitric oxide (NO) synthase (eNOS) and NO generation in VEGF-treated HUVECs. Both VEGF-induced NO and reactive oxygen species generation was involved in the inhibitory effect of FIR. Nitrotyrosine formation significantly increased in HUVECs treated with VEGF and FIR together. Inhibition of phosphoinositide 3-kinase (PI3K) by wortmannin abolished the FIR-induced phosphorylation of eNOS and Akt in HUVECs. FIR exposure upregulated the expression of PI3K p85 at the transcriptional level. We further found that FIR exposure induced the nuclear translocation of promyelocytic leukemia zinc finger protein (PLZF) in HUVECs. This induction was independent of a thermal effect. The small interfering RNA transfection of PLZF blocked FIR-increased PI3K levels and the inhibitory effect of FIR. These data suggest that FIR induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in HUVECs.
Conflict of interest statement
Figures
References
-
- Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–1088. - PubMed
-
- Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, et al. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ J. 2005;69:722–729. - PubMed
-
- Kihara T, Biro S, Ikeda Y, Fukudome T, Shinsato T, et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J. 2004;68:1146–1151. - PubMed
-
- Akasaki Y, Miyata M, Eto H, Shirasawa T, Hamada N, et al. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J. 2006;70:463–470. - PubMed
-
- Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, et al. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006;22:78–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

