Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial
- PMID: 22292061
- PMCID: PMC3264625
- DOI: 10.1371/journal.pone.0030856
Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial
Abstract
Objective: To explore the best interventions and working patterns of clinical pharmacists in pediatrics and to determine the effectiveness of clinical pharmacists in pediatrics.
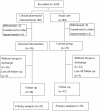
Methods: We conducted a randomized controlled trial of 160 pediatric patients with nerve system disease, respiratory system disease or digestive system disease, who were randomly allocated into two groups, with 80 in each group. Interventions by clinical pharmacists in the experimental group included answering questions of physicians and nurses, giving advice on treating patients, checking prescriptions and patient counseling at discharge. In the control group, patients were treated without clinical pharmacist interventions.
Results: Of the 109 interventions provided by clinical pharmacists during 4 months, 47 were consultations for physicians and nurses, 31 were suggestions of treatment, with 30 accepted by physicians (96.77%) and 31 were medical errors found in 641 prescriptions. Five adverse drug reactions were submitted to the adverse drug reaction monitoring network, with three in the experimental group and two in the control group. The average length of stay (LOS) for patients with respiratory system diseases in the experimental group was 6.45 days, in comparison with 10.83 days in the control group, which was statistically different (p value<0.05); Average drug compliance rate in the experimental group was 81.41%, in comparison with 70.17% of the control group, which was statistically different (p value<0.05). Cost of drugs and hospitalization and rate of readmission in two weeks after discharge in the two groups were not statistically different.
Conclusion: Participation by clinical pharmacists in the pharmacotherapy of pediatric patients can reduce LOS of patients with respiratory system disease and improve compliance rate through discharge education, showing no significant effects on prevention of ADR, reduction of cost of drugs and hospitalization and readmission rate in two weeks.
Trial registration: Chinese Clinical Trial Registry ChiCTR-TRC-10001081.
Conflict of interest statement
References
-
- Promoting safety of medicines for children [EB/OL]. www.who.com 2010-1-20.
-
- Wong ICK, Ghaleb MA, Franklin-Dean B, Barber N. Incidence and nature of dosing errors in pediatric medications. Drug Safety. 2004;27:661–70. - PubMed
-
- Academic Health Centers. Leading Change in the 21st Century. Washington, DC: National Academy Press; 2003.
-
- Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human. Washington, DC: National Academy Press; 2000. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials