The HIV1 protein Vpr acts to enhance constitutive DCAF1-dependent UNG2 turnover
- PMID: 22292079
- PMCID: PMC3265533
- DOI: 10.1371/journal.pone.0030939
The HIV1 protein Vpr acts to enhance constitutive DCAF1-dependent UNG2 turnover
Abstract
Background: The HIV1 protein Vpr assembles with and acts through an ubiquitin ligase complex that includes DDB1 and cullin 4 (CRL4) to cause G2 cell cycle arrest and to promote degradation of both uracil DNA glycosylase 2 (UNG2) and single-strand selective mono-functional uracil DNA glycosylase 1 (SMUG1). DCAF1, an adaptor protein, is required for Vpr-mediated G2 arrest through the ubiquitin ligase complex. In work described here, we used UNG2 as a model substrate to study how Vpr acts through the ubiquitin ligase complex. We examined whether DCAF1 is essential for Vpr-mediated degradation of UNG2 and SMUG1. We further investigated whether Vpr is required for recruiting substrates to the ubiquitin ligase or acts to enhance its function and whether this parallels Vpr-mediated G2 arrest.
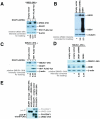
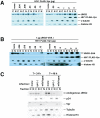
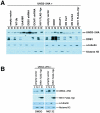
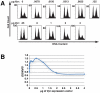
Methodology/principal findings: We found that DCAF1 plays an important role in Vpr-independent UNG2 and SMUG1 depletion. UNG2 assembled with the ubiquitin ligase complex in the absence of Vpr, but Vpr enhanced this interaction. Further, Vpr-mediated enhancement of UNG2 degradation correlated with low Vpr expression levels. Vpr concentrations exceeding a threshold blocked UNG2 depletion and enhanced its accumulation in the cell nucleus. A similar dose-dependent trend was seen for Vpr-mediated cell cycle arrest.
Conclusions/significance: This work identifies UNG2 and SMUG1 as novel targets for CRL4(DCAF1)-mediated degradation. It further shows that Vpr enhances rather than enables the interaction between UNG2 and the ubiquitin ligase. Vpr augments CRL4(DCAF1)-mediated UNG2 degradation at low concentrations but antagonizes it at high concentrations, allowing nuclear accumulation of UNG2. Further, the protein that is targeted to cause G2 arrest behaves much like UNG2. Our findings provide the basis for determining whether the CRL4(DCAF1) complex is alone responsible for cell cycle-dependent UNG2 turnover and will also aid in establishing conditions necessary for the identification of additional targets of Vpr-enhanced degradation.
Conflict of interest statement
Figures
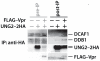


References
-
- Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282:27046–27057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

