Anti-inflammatory role of fetuin-A in injury and infection
- PMID: 22292896
- PMCID: PMC3349766
- DOI: 10.2174/156652412800620039
Anti-inflammatory role of fetuin-A in injury and infection
Abstract
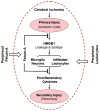
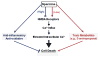
Infection and injury are two seemingly unrelated processes that often converge on common innate inflammatory responses mediated by pathogen- or damage-associated molecular patterns (PAMPs or DAMPs). If dysregulated, an excessive inflammation manifested by the overproduction and release of proinflammatory mediators (e.g., TNF, IFN-γ, and HMGB1) may adversely lead to many pathogenic consequences. As a counter-regulatory mechanism, the liver strategically re-prioritizes the synthesis and systemic release of acute phase proteins (APP) including the fetuin-A (also termed alpha-2-HS-glycoprotein for the human homologue). Fetuin-A is divergently regulated by different proinflammatory mediators, and functions as a positive or negative APP in injury and infection. It not only facilitates anti-inflammatory actions of cationic polyamines (e.g., spermine), but also directly inhibits PAMP-induced HMGB1 release by innate immune cells. Peripheral administration of fetuin-A promotes a short-term reduction of cerebral ischemic injury, but confers a long-lasting protection against lethal endotoxemia. Furthermore, delayed administration of fetuin-A rescues mice from lethal sepsis even when the first dose is given 24 hours post the onset of disease. Collectively, these findings have reinforced an essential role for fetuin-A in counter-regulating injury- or infection-elicited inflammatory responses.
Figures

References
-
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285(5428):732–736. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. - PubMed
-
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
