Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases
- PMID: 22293182
- PMCID: PMC3266780
- DOI: 10.1172/JCI57416
Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases
Abstract
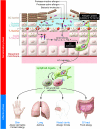
Classic atopic dermatitis is complicated by asthma, allergic rhinitis, and food allergies, cumulatively referred to as atopic diseases. Recent discoveries of mutations in the filaggrin gene as predisposing factors for atopic diseases have refocused investigators' attention on epidermal barrier dysfunction as a causative mechanism. The skin's barrier function has three elements: the stratum corneum (air-liquid barrier), tight junctions (liquid-liquid barrier), and the Langerhans cell network (immunological barrier). Clarification of the molecular events underpinning epidermal barrier function and dysfunction should lead to a better understanding of the pathophysiological mechanisms of atopic diseases.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

