Neuroprotective function for ramified microglia in hippocampal excitotoxicity
- PMID: 22293457
- PMCID: PMC3292937
- DOI: 10.1186/1742-2094-9-27
Neuroprotective function for ramified microglia in hippocampal excitotoxicity
Abstract
Background: Most of the known functions of microglia, including neurotoxic and neuroprotective properties, are attributed to morphologically-activated microglia. Resting, ramified microglia are suggested to primarily monitor their environment including synapses. Here, we show an active protective role of ramified microglia in excitotoxicity-induced neurodegeneration.
Methods: Mouse organotypic hippocampal slice cultures were treated with N-methyl-D-aspartic acid (NMDA) to induce excitotoxic neuronal cell death. This procedure was performed in slices containing resting microglia or slices that were chemically or genetically depleted of their endogenous microglia.
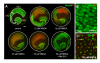
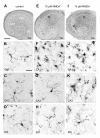
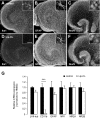
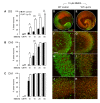
Results: Treatment of mouse organotypic hippocampal slice cultures with 10-50 μM N-methyl-D-aspartic acid (NMDA) induced region-specific excitotoxic neuronal cell death with CA1 neurons being most vulnerable, whereas CA3 and DG neurons were affected less. Ablation of ramified microglia severely enhanced NMDA-induced neuronal cell death in the CA3 and DG region rendering them almost as sensitive as CA1 neurons. Replenishment of microglia-free slices with microglia restored the original resistance of CA3 and DG neurons towards NMDA.
Conclusions: Our data strongly suggest that ramified microglia not only screen their microenvironment but additionally protect hippocampal neurons under pathological conditions. Morphological activation of ramified microglia is thus not required to influence neuronal survival.
Figures

References
-
- Van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

